Molecular Metabolism ( IF 7.0 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.molmet.2020.101091 Stewart W C Masson 1 , Brie Sorrenson 2 , Peter R Shepherd 3 , Troy L Merry 1
|
Objective
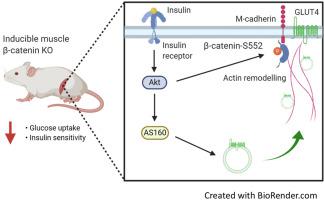
Skeletal muscle glucose disposal following a meal is mediated through insulin-stimulated movement of the GLUT4-containing vesicles to the cell surface. The highly conserved scaffold-protein β-catenin is an emerging regulator of vesicle trafficking in other tissues. Here, we investigated the involvement of β-catenin in skeletal muscle insulin-stimulated glucose transport.
Methods
Glucose homeostasis and transport was investigated in inducible muscle specific β-catenin knockout (BCAT-mKO) mice. The effect of β-catenin deletion and mutation of β-catenin serine 552 on signal transduction, glucose uptake and protein–protein interactions were determined in L6-G4-myc cells, and β-catenin insulin-responsive binding partners were identified via immunoprecipitation coupled to label-free proteomics.
Results
Skeletal muscle specific deletion of β-catenin impaired whole-body insulin sensitivity and insulin-stimulated glucose uptake into muscle independent of canonical Wnt signalling. In response to insulin, β-catenin was phosphorylated at serine 552 in an Akt-dependent manner, and in L6-G4-myc cells, mutation of β-cateninS552 impaired insulin-induced actin-polymerisation, resulting in attenuated insulin-induced glucose transport and GLUT4 translocation. β-catenin was found to interact with M-cadherin in an insulin-dependent β-cateninS552-phosphorylation dependent manner, and loss of M-cadherin in L6-G4-myc cells attenuated insulin-induced actin-polymerisation and glucose transport.
Conclusions
Our data suggest that β-catenin is a novel mediator of glucose transport in skeletal muscle and may contribute to insulin-induced actin-cytoskeleton remodelling to support GLUT4 translocation.
中文翻译:

β-连环蛋白通过肌动蛋白重塑和 M-钙粘蛋白结合调节肌肉葡萄糖转运
客观的
餐后骨骼肌葡萄糖的处理是通过含有 GLUT4 的囊泡向细胞表面的胰岛素刺激运动介导的。高度保守的支架蛋白 β-连环蛋白是其他组织中囊泡运输的新兴调节剂。在这里,我们研究了 β-catenin 参与骨骼肌胰岛素刺激的葡萄糖转运。
方法
在诱导型肌肉特异性 β-连环蛋白敲除 (BCAT-mKO) 小鼠中研究了葡萄糖稳态和转运。在 L6-G4-myc 细胞中测定了 β-连环蛋白缺失和 β-连环蛋白丝氨酸 552 突变对信号转导、葡萄糖摄取和蛋白质-蛋白质相互作用的影响,并通过免疫沉淀偶联鉴定了 β-连环蛋白胰岛素反应性结合伴侣到无标记蛋白质组学。
结果
β-连环蛋白的骨骼肌特异性缺失会损害全身胰岛素敏感性和胰岛素刺激的肌肉葡萄糖摄取,而与经典 Wnt 信号无关。响应胰岛素,β-连环蛋白以 Akt 依赖性方式在丝氨酸 552 处磷酸化,并且在 L6-G4-myc 细胞中,β-连环蛋白S552 的突变削弱了胰岛素诱导的肌动蛋白聚合,导致胰岛素诱导的葡萄糖减弱运输和 GLUT4 易位。发现 β-连环蛋白以胰岛素依赖性 β-连环蛋白S552磷酸化依赖性方式与 M-钙粘蛋白相互作用,并且 L6-G4-myc 细胞中 M-钙粘蛋白的缺失减弱了胰岛素诱导的肌动蛋白聚合和葡萄糖转运。
结论
我们的数据表明,β-连环蛋白是骨骼肌中葡萄糖转运的新型介质,可能有助于胰岛素诱导的肌动蛋白-细胞骨架重塑以支持 GLUT4 易位。









































 京公网安备 11010802027423号
京公网安备 11010802027423号