Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2020-10-02 , DOI: 10.1016/j.jmgm.2020.107771 Son Tung Ngo 1 , Van Van Vu 2 , Huong Thi Thu Phung 2
|
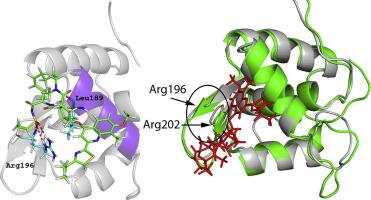
The methyl methanesulfonate and ultraviolet sensitive 81 (MUS81) is a structure-specific endonuclease that is highly conserved in eukaryotes and essential for homologous recombination repair. The winged-helix domain at the N-terminus of MUS81 (wMUS81) can bind DNA substrates and regulate the endonuclease activity. The repression of MUS81 activity could enhance the sensitivity to antitumor compounds of different tumour cells. Thus, MUS81 is a potential therapeutic target in cancer therapy. However, specific inhibitors of MUS81 have remained elusive. Here, for the first time, we attempt to discover the compounds disrupting the wMUS81 activity. The binding affinity of available drugs to wMUS81 was first estimated by molecular docking. pKa values were taken into consideration to eliminate unlikely protonation states of the ligands. Top-lead compounds were then estimated the binding affinity using the fast pulling ligand simulations. Finally, the free energy perturbation method accurately defined the absolute binding free energy of the top four ligands, revealing the most potential inhibitors of wMUS81 including simeprevir and nilotinib. Binding of simeprevir destabilizes the β-hairpin region of wMUS81, likely disturbing the wMUS81 function. The van der Waals free binding energy majorly modulates the ligand-binding mechanism. The two conserved residues Leu189 and Arg196 are likely important in monitoring the interacting process of simeprevir to wMUS81.
中文翻译:

MUS81的翼螺旋结构域的可能抑制剂的计算研究
甲磺酸甲酯和紫外线敏感性81(MUS81)是一种结构特异性核酸内切酶,在真核生物中高度保守,对于同源重组修复至关重要。MUS81(w MUS81)N末端的翼状螺旋结构域可以结合DNA底物并调节核酸内切酶活性。MUS81活性的抑制可以增强对不同肿瘤细胞的抗肿瘤化合物的敏感性。因此,MUS81是癌症治疗中潜在的治疗靶标。但是,MUS81的特异性抑制剂仍然难以捉摸。在这里,我们首次尝试发现破坏w MUS81活性的化合物。首先通过分子对接估计可用药物与w MUS81的结合亲和力。K考虑了一个值以消除配体的不太可能的质子化状态。然后使用快速拉动配体模拟法估计前导化合物的结合亲和力。最后,自由能的扰动方法精确限定的前四位配体的绝对结合自由能,露出的最有潜力的抑制剂瓦特MUS81包括simeprevir和尼洛替尼。simeprevir的结合使wMUS81的β-发夹区不稳定,可能干扰wMUS81功能。范德华力的自由结合能主要调节配体结合机理。这两个保守残基Leu189和Arg196在监测simeprevir与w MUS81的相互作用过程中可能很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号