Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.chembiol.2020.09.004 Yusaku Miyamae 1 , Ling-Chun Chen 2 , Yuki Utsugi 3 , Helen Farrants 2 , Thomas J Wandless 2
|
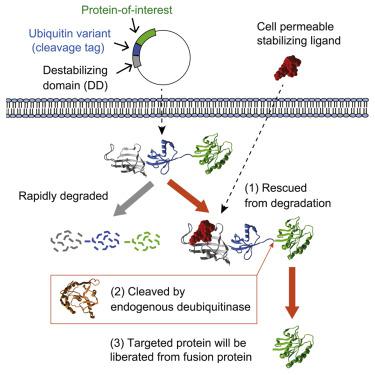
Here, we report a method to regulate cellular protein levels by introducing a ubiquitin variant between a destabilizing domain (DD) and the regulated protein. When produced in the absence of a stabilizing ligand the DD dominates and the entire fusion protein is processively degraded by the proteasome. In the presence of the stabilizing ligand the fusion protein is metabolically stable and becomes a substrate for abundant ubiquitin-specific proteases, liberating a native, or a near-native protein-of-interest. This technique is thus particularly useful for the study of proteins whose free N terminus is required for proper function. In addition, removal of the DD in the presence of stabilizing ligand leads to higher expression levels of regulated protein when cells experience transient exposure to a stabilizing ligand, such as in a living animal receiving a single dose of a pharmacological agent as the stabilizing ligand.
中文翻译:

一种以天然或近天然形式有条件调节蛋白质稳定性的方法
在这里,我们报告了一种通过在不稳定结构域 (DD) 和受调节蛋白质之间引入泛素变体来调节细胞蛋白质水平的方法。当在没有稳定配体的情况下产生时,DD 占主导地位,并且整个融合蛋白被蛋白酶体逐步降解。在稳定配体存在的情况下,融合蛋白在代谢上是稳定的,并成为大量泛素特异性蛋白酶的底物,从而释放出天然或接近天然的目标蛋白质。因此,该技术对于研究其正常功能需要游离 N 末端的蛋白质特别有用。此外,当细胞短暂暴露于稳定配体时,在稳定配体存在的情况下去除 DD 会导致调节蛋白的更高表达水平,











































 京公网安备 11010802027423号
京公网安备 11010802027423号