Catalysis Today ( IF 5.2 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.cattod.2020.09.020 Christina H.M. van Oversteeg , Marisol Tapia Rosales , Kristiaan H. Helfferich , Mahnaz Ghiasi , Johannes D. Meeldijk , Nienke J. Firet , Peter Ngene , Celso de Mello Donegá , Petra E. de Jongh
|
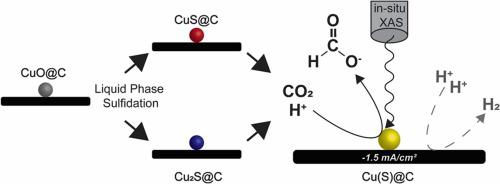
The electrocatalytic reduction of CO2 to produce sustainable fuels and chemicals is attracting great attention. Cu-based catalysts can lead to the production of a range of different molecules, and interestingly the product selectivity strongly depends on the preparation history, although it is not fully understood yet why. We report a novel strategy that allowed us to prepare Cu nanoparticle on carbon catalysts with similar morphologies, but prepared by in-situ reduction of either supported CuS, Cu2S or CuO nanoparticles. For the first time the evolution of the Cu species was followed under CO2 and H+ reduction conditions using in-situ X-ray absorption spectroscopy. Excellent electrochemical contact between the Cu-based nanoparticles, the carbon support and the carbon-paper substrate was observed, resulting in metallic Cu as the predominant phase under typical electrochemical CO2 reduction conditions. Even covering less than 4% of the H2 producing carbon support with Cu-sulfide derived nanoparticles allowed to steer the selectivity to a maximum of 12% Faradaic efficiency for the production of formate. Clear differences between the catalysts derived from CuS, Cu2S or CuO nanoparticles were observed, which was ascribed to the presence of residual sulfur in the catalysts.
中文翻译:

用于电化学还原二氧化碳的碳负载硫化铜衍生纳米颗粒
用于生产可持续燃料和化学品的 CO 2电催化还原引起了极大的关注。铜基催化剂可以产生一系列不同的分子,有趣的是,产物选择性在很大程度上取决于制备历史,尽管尚不完全了解原因。我们报告了一种新策略,该策略允许我们在具有相似形态的碳催化剂上制备 Cu 纳米颗粒,但通过原位还原负载的 CuS、Cu 2 S 或 CuO 纳米颗粒来制备。首次在 CO 2和 H +下跟踪了 Cu 物种的演变使用原位 X 射线吸收光谱的还原条件。观察到Cu基纳米颗粒、碳载体和碳纸基材之间的优异电化学接触,导致在典型的电化学CO 2还原条件下金属Cu作为主要相。即使用硫化铜衍生的纳米粒子覆盖不到 4% 的产生 H 2 的碳载体,也允许将选择性控制到最大 12% 的法拉第效率以生产甲酸盐。观察到衍生自 CuS、Cu 2 S 或 CuO 纳米颗粒的催化剂之间存在明显差异,这归因于催化剂中残留硫的存在。










































 京公网安备 11010802027423号
京公网安备 11010802027423号