Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.bbagen.2020.129756 Ning-lin Zhao , Qian-qian Zhang , Chang Zhao , Li Liu , Tao Li , Chang-cheng Li , Li-hui He , Yi-bo Zhu , Ying-jie Song , Huan-xiang Liu , Rui Bao
|
Background
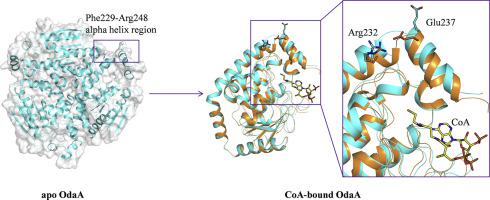
Crotonase superfamily members exhibit great catalytic diversity towards various acyl-CoA substrates. A common CoA moiety binding pattern is usually observed in this family, understanding the substrate-binding mechanism would facilitate the rational engineering of crotonases for improved properties.
Methods
We applied X-ray crystallography to investigate a putative enoyl-CoA hydratase/isomerase OdaA in Pseudomonas aeruginosa. Thermal shift assay (TSA) were performed to explore the binding of OdaA with CoA thioester substrates. Furthermore, we performed molecular dynamics (MD) simulations to elucidate the dynamics of its CoA-binding site.
Results
We solved the crystal structures of the apo and CoA-bound OdaA. Thermal shift assay (TSA) showed that CoA thioester substrates bind to OdaA with a different degree. MD simulations demonstrated that the C-terminal alpha helix underwent a structural transition and a hinge region would associate with this conformational change.
Conclusions
TSA in combination with MD simulations elucidate that the dynamics of C-terminal alpha helix in CoA-binding, and a hinge region play an important role in conformational change.
General significance
Those results help to extend our knowledge about the nature of crotonases and would be informative for future mechanistic studies and industry applications.
中文翻译:

铜绿假单胞菌OdaA的结构和分子动力学研究揭示了C末端铰链元件的调节作用
背景
巴豆酶超家族成员对各种酰基辅酶A底物表现出很大的催化多样性。通常在该家族中观察到常见的CoA部分结合模式,了解底物结合机制将有助于对巴豆酶进行合理的改造,以改善其性能。
方法
我们应用X射线晶体学研究了铜绿假单胞菌中假定的烯酰辅酶A水合酶/异构酶OdaA 。进行热位移测定(TSA)以研究OdaA与CoA硫酯底物的结合。此外,我们进行了分子动力学(MD)模拟,以阐明其CoA结合位点的动力学。
结果
我们解决了载脂蛋白和结合CoA的OdaA的晶体结构。热移分析(TSA)显示,CoA硫酯底物以不同程度结合到OdaA。MD模拟表明,C末端的α螺旋经历了结构转变,而铰链区将与此构象变化相关。
结论
TSA与MD模拟相结合,阐明了CoA结合中C末端α螺旋的动力学以及铰链区在构象变化中起着重要作用。
一般意义
这些结果有助于扩展我们对巴豆酶性质的了解,并为将来的机理研究和工业应用提供信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号