当前位置:
X-MOL 学术
›
Appl. Geochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent extraction of lithium from simulated shale gas produced water with a bifunctional ionic liquid
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.apgeochem.2020.104783 Guillaume Zante , Dominique Trébouet , Maria Boltoeva
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.apgeochem.2020.104783 Guillaume Zante , Dominique Trébouet , Maria Boltoeva
|
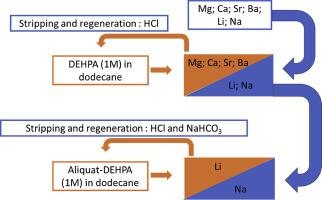
Abstract The recovery of lithium from brines is a major field of study with an increase in lithium-ion batteries consumption and the subsequent growth of lithium consumption. The recovery of lithium from shale gas produced water is promising since these sources could contain non-negligible concentrations of lithium. In this study, lithium extraction was investigated using solvent extraction with a bifunctional ionic liquid (IL) as an extracting agent diluted in n-dodecane. The components of these IL are cheap and commercially available products, namely Aliquat-336 (methyltrioctylammonium chloride) and DEHPA (di-(2-ethylhexyl)phosphoric acid), and its synthesis is straightforward. Lithium extraction was optimized by studying several experimental parameters (mixing time, aqueous phase acidity, IL concentration in the solvent phase, aqueous lithium concentration). The mechanism of extraction was detailed, and the stripping was shown to be complete with 0.5 mol.L−1 of HCl. A two stages strategy was defined to recover lithium from synthetic brine. In the first stage, divalent metals are removed using five successive cycles of extraction with DEHPA (1 mol.L−1) dissolved in n-dodecane. In the second stage, the IL extracting agent [Aliquat-336][DEHPA] (1 mol.L−1) allowed to remove 83% of lithium in one cycle of extraction, which is higher than reported solvent extraction results with conventional extracting molecules.
中文翻译:

双功能离子液体从模拟页岩气采出水中溶剂萃取锂
摘要 随着锂离子电池消耗量的增加和随后锂消耗量的增长,从卤水中回收锂是一个主要的研究领域。从页岩气采出水中回收锂很有前景,因为这些来源可能含有不可忽视的锂浓度。在本研究中,使用溶剂萃取法对锂萃取进行了研究,双官能离子液体 (IL) 作为萃取剂在正十二烷中稀释。这些 IL 的成分是廉价且可商购的产品,即 Aliquat-336(甲基三辛基氯化铵)和 DEHPA(二-(2-乙基己基)磷酸),其合成很简单。通过研究几个实验参数(混合时间、水相酸度、溶剂相中的离子液体浓度、含水锂浓度)。详细的萃取机理,表明使用 0.5 mol.L-1 HCl 即可完成汽提。定义了从合成盐水中回收锂的两阶段策略。在第一阶段,使用溶解在正十二烷中的 DEHPA (1 mol.L-1) 进行五次连续萃取循环以去除二价金属。在第二阶段,离子液体萃取剂 [Aliquat-336][DEHPA] (1 mol.L−1) 在一次萃取循环中可以去除 83% 的锂,高于传统萃取分子的溶剂萃取结果. 使用溶解在正十二烷中的 DEHPA (1 mol.L-1) 进行五次连续萃取循环,去除二价金属。在第二阶段,离子液体萃取剂 [Aliquat-336][DEHPA] (1 mol.L−1) 在一次萃取循环中可以去除 83% 的锂,高于传统萃取分子的溶剂萃取结果. 使用溶解在正十二烷中的 DEHPA (1 mol.L-1) 进行五次连续萃取循环,去除二价金属。在第二阶段,离子液体萃取剂 [Aliquat-336][DEHPA] (1 mol.L−1) 在一次萃取循环中可以去除 83% 的锂,高于传统萃取分子的溶剂萃取结果.
更新日期:2020-12-01
中文翻译:

双功能离子液体从模拟页岩气采出水中溶剂萃取锂
摘要 随着锂离子电池消耗量的增加和随后锂消耗量的增长,从卤水中回收锂是一个主要的研究领域。从页岩气采出水中回收锂很有前景,因为这些来源可能含有不可忽视的锂浓度。在本研究中,使用溶剂萃取法对锂萃取进行了研究,双官能离子液体 (IL) 作为萃取剂在正十二烷中稀释。这些 IL 的成分是廉价且可商购的产品,即 Aliquat-336(甲基三辛基氯化铵)和 DEHPA(二-(2-乙基己基)磷酸),其合成很简单。通过研究几个实验参数(混合时间、水相酸度、溶剂相中的离子液体浓度、含水锂浓度)。详细的萃取机理,表明使用 0.5 mol.L-1 HCl 即可完成汽提。定义了从合成盐水中回收锂的两阶段策略。在第一阶段,使用溶解在正十二烷中的 DEHPA (1 mol.L-1) 进行五次连续萃取循环以去除二价金属。在第二阶段,离子液体萃取剂 [Aliquat-336][DEHPA] (1 mol.L−1) 在一次萃取循环中可以去除 83% 的锂,高于传统萃取分子的溶剂萃取结果. 使用溶解在正十二烷中的 DEHPA (1 mol.L-1) 进行五次连续萃取循环,去除二价金属。在第二阶段,离子液体萃取剂 [Aliquat-336][DEHPA] (1 mol.L−1) 在一次萃取循环中可以去除 83% 的锂,高于传统萃取分子的溶剂萃取结果. 使用溶解在正十二烷中的 DEHPA (1 mol.L-1) 进行五次连续萃取循环,去除二价金属。在第二阶段,离子液体萃取剂 [Aliquat-336][DEHPA] (1 mol.L−1) 在一次萃取循环中可以去除 83% 的锂,高于传统萃取分子的溶剂萃取结果.











































 京公网安备 11010802027423号
京公网安备 11010802027423号