当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic hydrogen atom transfer to alkenes: a roadmap for metal hydrides and radicals
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-29 , DOI: 10.1039/d0sc04112b Sophia L Shevick 1 , Conner V Wilson 2 , Simona Kotesova 1 , Dongyoung Kim 2 , Patrick L Holland 2 , Ryan A Shenvi 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-29 , DOI: 10.1039/d0sc04112b Sophia L Shevick 1 , Conner V Wilson 2 , Simona Kotesova 1 , Dongyoung Kim 2 , Patrick L Holland 2 , Ryan A Shenvi 1
Affiliation
|
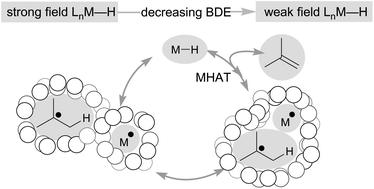
Hydrogen atom transfer from a metal hydride (MHAT) has emerged as a powerful, if puzzling, technique in chemical synthesis. In catalytic MHAT reactions, earth-abundant metal complexes generate stabilized and unstabilized carbon-centered radicals from alkenes of various substitution patterns with robust chemoselectivity. This perspective combines organic and inorganic perspectives to outline challenges and opportunities, and to propose working models to assist further developments. We attempt to demystify the putative intermediates, the basic elementary steps, and the energetic implications, especially for cage pair formation, collapse and separation. Distinctions between catalysts with strong-field (SF) and weak-field (WF) ligand environments may explain some differences in reactivity and selectivity, and provide an organizing principle for kinetics that transcends the typical thermodynamic analysis. This blueprint should aid practitioners who hope to enter and expand this exciting area of chemistry.
中文翻译:

催化氢原子转移到烯烃:金属氢化物和自由基的路线图
金属氢化物 (MHAT) 的氢原子转移已成为化学合成中一种强大但令人费解的技术。在催化 MHAT 反应中,富含地球的金属配合物从具有强大化学选择性的各种取代模式的烯烃生成稳定和不稳定的碳中心自由基。这种观点结合有机和无机观点来概述挑战和机遇,并提出工作模式以协助进一步发展。我们试图揭开假定的中间体、基本的基本步骤和能量影响的神秘面纱,特别是对于笼对的形成、塌陷和分离。具有强场(SF)和弱场(WF)配体环境的催化剂之间的区别可以解释反应性和选择性的一些差异,并提供超越典型热力学分析的动力学组织原理。该蓝图应有助于希望进入和扩展这一令人兴奋的化学领域的从业者。
更新日期:2020-10-08
中文翻译:

催化氢原子转移到烯烃:金属氢化物和自由基的路线图
金属氢化物 (MHAT) 的氢原子转移已成为化学合成中一种强大但令人费解的技术。在催化 MHAT 反应中,富含地球的金属配合物从具有强大化学选择性的各种取代模式的烯烃生成稳定和不稳定的碳中心自由基。这种观点结合有机和无机观点来概述挑战和机遇,并提出工作模式以协助进一步发展。我们试图揭开假定的中间体、基本的基本步骤和能量影响的神秘面纱,特别是对于笼对的形成、塌陷和分离。具有强场(SF)和弱场(WF)配体环境的催化剂之间的区别可以解释反应性和选择性的一些差异,并提供超越典型热力学分析的动力学组织原理。该蓝图应有助于希望进入和扩展这一令人兴奋的化学领域的从业者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号