当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthetic, Mechanistic and Biological Interrogation of Ginkgo biloba Chemical Space en route to (–)-Bilobalide
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-29 , DOI: 10.1021/jacs.0c08231 Robert M Demoret 1 , Meghan A Baker 1 , Masaki Ohtawa 1 , Shuming Chen 2 , Ching Ching Lam 2 , Sophia Khom 3 , Marisa Roberto 3 , Stefano Forli 4 , Kendall N Houk 2 , Ryan A Shenvi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-09-29 , DOI: 10.1021/jacs.0c08231 Robert M Demoret 1 , Meghan A Baker 1 , Masaki Ohtawa 1 , Shuming Chen 2 , Ching Ching Lam 2 , Sophia Khom 3 , Marisa Roberto 3 , Stefano Forli 4 , Kendall N Houk 2 , Ryan A Shenvi 1
Affiliation
|
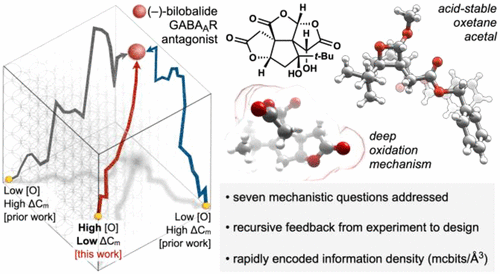
Here we interrogate the structurally dense (1.63 mcbits/Å3) GABAA receptor antagonist bilobalide, intermediates en route to its synthesis and related mechanistic questions. 13C isotope labeling identifies an unexpected bromine migration en route to an α-selective, catalytic asymmetric Reformatsky reaction, ruling out an asymmetric allylation pathway. Experiment and computation converge on the driving forces behind two surprising observations. First, an oxetane acetal persists in concen-trated mineral acid (1.5 M DCl in THF-d8/D2O); its longevity is correlated to destabilizing steric clash between substituents upon ring-opening. Second, a regioselective oxidation of des-hydroxybilobalide is found to rely on lactone acidification through lone-pair delocalization, which leads to extremely rapid intermolecular enolate equilibration. We also establish equivalent effects of (-)-bilobalide and the nonconvulsive sesquiterpene (-)-jiadifenolide on action potential-independent in-hibitory currents at GABAergic synapses, using (+)-bilobalide as a negative control. The high information density of bilob-alide distinguishes it from other scaffolds, and may characterize natural product (NP) space more generally. Therefore, we also include a Python script to quickly (ca. 132,000 molecules/ minute) calculate information content (Böttcher scores), which may prove helpful to identify important features of NP space.
中文翻译:

银杏叶化学空间在生成 (–)-Bilobalide 的过程中的合成、机理和生物学研究
在这里,我们探讨了结构致密 (1.63 mcbits/Å3) GABAA 受体拮抗剂白叶内酯、其合成过程中的中间体以及相关的机制问题。 13C 同位素标记可识别出在 α 选择性催化不对称 Reformatsky 反应途中发生的意外溴迁移,从而排除了不对称烯丙基化途径。实验和计算集中于两个令人惊讶的观察结果背后的驱动力。首先,氧杂环丁烷缩醛在浓无机酸中持久存在(1.5 M DCl,溶于 THF-d8/D2O);其寿命与开环时取代基之间不稳定的空间冲突有关。其次,发现脱羟基白果内酯的区域选择性氧化依赖于通过孤对离域的内酯酸化,这导致极快的分子间烯醇化物平衡。我们还使用 (+)-白果内酯作为阴性对照,建立了 (-)-白果内酯和非惊厥性倍半萜 (-)-jiadifenolide 对 GABA 能突触的动作电位独立抑制电流的等效作用。 bilob-alide 的高信息密度使其有别于其他支架,并且可以更普遍地表征天然产物(NP)空间。因此,我们还包含一个 Python 脚本来快速(约 132,000 个分子/分钟)计算信息内容(Böttcher 分数),这可能有助于识别 NP 空间的重要特征。
更新日期:2020-09-29
中文翻译:

银杏叶化学空间在生成 (–)-Bilobalide 的过程中的合成、机理和生物学研究
在这里,我们探讨了结构致密 (1.63 mcbits/Å3) GABAA 受体拮抗剂白叶内酯、其合成过程中的中间体以及相关的机制问题。 13C 同位素标记可识别出在 α 选择性催化不对称 Reformatsky 反应途中发生的意外溴迁移,从而排除了不对称烯丙基化途径。实验和计算集中于两个令人惊讶的观察结果背后的驱动力。首先,氧杂环丁烷缩醛在浓无机酸中持久存在(1.5 M DCl,溶于 THF-d8/D2O);其寿命与开环时取代基之间不稳定的空间冲突有关。其次,发现脱羟基白果内酯的区域选择性氧化依赖于通过孤对离域的内酯酸化,这导致极快的分子间烯醇化物平衡。我们还使用 (+)-白果内酯作为阴性对照,建立了 (-)-白果内酯和非惊厥性倍半萜 (-)-jiadifenolide 对 GABA 能突触的动作电位独立抑制电流的等效作用。 bilob-alide 的高信息密度使其有别于其他支架,并且可以更普遍地表征天然产物(NP)空间。因此,我们还包含一个 Python 脚本来快速(约 132,000 个分子/分钟)计算信息内容(Böttcher 分数),这可能有助于识别 NP 空间的重要特征。









































 京公网安备 11010802027423号
京公网安备 11010802027423号