当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Delineating Heme-Mediated versus Direct Protein Oxidation in Peroxidase-Activated Cytochrome c by Top-Down Mass Spectrometry
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-29 , DOI: 10.1021/acs.biochem.0c00609 Victor Yin 1 , Derek Holzscherer 1 , Lars Konermann 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-09-29 , DOI: 10.1021/acs.biochem.0c00609 Victor Yin 1 , Derek Holzscherer 1 , Lars Konermann 1
Affiliation
|
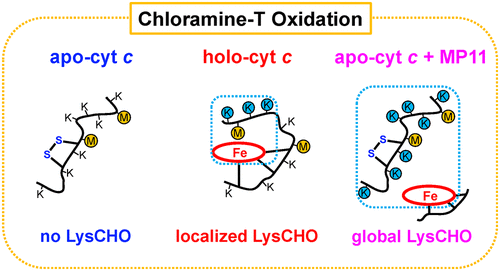
Oxidation of key residues in cytochrome c (cyt c) by chloramine T (CT) converts the protein from an electron transporter to a peroxidase. This peroxidase-activated state represents an important model system for exploring the early steps of apoptosis. CT-induced transformations include oxidation of the distal heme ligand Met80 (MetO, +16 Da) and carbonylation (LysCHO, −1 Da) in the range of Lys53/55/72/73. Remarkably, the 15 remaining Lys residues in cyt c are not susceptible to carbonylation. The cause of this unusual selectivity is unknown. Here we applied top-down mass spectrometry (MS) to examine whether CT-induced oxidation is catalyzed by heme. To this end, we compared the behavior of cyt c with (holo-cyt c) and without heme (apoSS-cyt c). CT caused MetO formation at Met80 for both holo- and apoSS-cyt c, implying that this transformation can proceed independently of heme. The aldehyde-specific label Girard’s reagent T (GRT) reacted with oxidized holo-cyt c, consistent with the presence of several LysCHO. In contrast, oxidized apo-cyt c did not react with GRT, revealing that LysCHO forms only in the presence of heme. The heme dependence of LysCHO formation was further confirmed using microperoxidase-11 (MP11). CT exposure of apoSS-cyt c in the presence of MP11 caused extensive nonselective LysCHO formation. Our results imply that the selectivity of LysCHO formation at Lys53/55/72/73 in holo-cyt c is caused by the spatial proximity of these sites to the reactive (distal) heme face. Overall, this work highlights the utility of top-down MS for unravelling complex oxidative modifications.
中文翻译:

通过自上而下的质谱图描绘过氧化物酶激活的细胞色素c中血红素介导的与直接蛋白质的氧化
氯胺T(CT)对细胞色素c(cyt c)中关键残基的氧化将蛋白质从电子转运体转化为过氧化物酶。这种过氧化物酶激活状态代表了探索细胞凋亡早期步骤的重要模型系统。CT诱导的转化包括远端血红素配体Met80(MetO,+16 Da)的氧化和羰基化(LysCHO,-1 Da)在Lys53 / 55/72/73范围内。值得注意的是,cyt c中剩余的15个Lys残基不易羰基化。这种异常选择性的原因尚不清楚。在这里,我们应用了自上而下的质谱(MS)来检查CT诱导的氧化是否被血红素催化。为此,我们将cyt c与(holo-cyt c)且不含血红素(Apo SS -cyt c)。CT导致完整的和脱辅基SS -cyt c在Met80处形成MetO ,这表明这种转化可以独立于血红素进行。醛特异性标记吉拉德试剂T(GRT)与氧化的全细胞c发生反应,这与几种LysCHO的存在一致。相比之下,氧化的载脂细胞c不与GRT反应,表明LysCHO仅在血红素存在下形成。使用微过氧化物酶11(MP11)进一步证实了LysCHO形成的血红素依赖性。Apo SS -cyt c的CT暴露在MP11存在下引起广泛的非选择性LysCHO形成。我们的结果表明,在全细胞c中Lys53 / 55/72/73处LysCHO形成的选择性是由于这些位点与反应性(远端)血红素面在空间上接近而引起的。总的来说,这项工作强调了自上而下的质谱仪可用于揭示复杂的氧化修饰。
更新日期:2020-10-28
中文翻译:

通过自上而下的质谱图描绘过氧化物酶激活的细胞色素c中血红素介导的与直接蛋白质的氧化
氯胺T(CT)对细胞色素c(cyt c)中关键残基的氧化将蛋白质从电子转运体转化为过氧化物酶。这种过氧化物酶激活状态代表了探索细胞凋亡早期步骤的重要模型系统。CT诱导的转化包括远端血红素配体Met80(MetO,+16 Da)的氧化和羰基化(LysCHO,-1 Da)在Lys53 / 55/72/73范围内。值得注意的是,cyt c中剩余的15个Lys残基不易羰基化。这种异常选择性的原因尚不清楚。在这里,我们应用了自上而下的质谱(MS)来检查CT诱导的氧化是否被血红素催化。为此,我们将cyt c与(holo-cyt c)且不含血红素(Apo SS -cyt c)。CT导致完整的和脱辅基SS -cyt c在Met80处形成MetO ,这表明这种转化可以独立于血红素进行。醛特异性标记吉拉德试剂T(GRT)与氧化的全细胞c发生反应,这与几种LysCHO的存在一致。相比之下,氧化的载脂细胞c不与GRT反应,表明LysCHO仅在血红素存在下形成。使用微过氧化物酶11(MP11)进一步证实了LysCHO形成的血红素依赖性。Apo SS -cyt c的CT暴露在MP11存在下引起广泛的非选择性LysCHO形成。我们的结果表明,在全细胞c中Lys53 / 55/72/73处LysCHO形成的选择性是由于这些位点与反应性(远端)血红素面在空间上接近而引起的。总的来说,这项工作强调了自上而下的质谱仪可用于揭示复杂的氧化修饰。







































 京公网安备 11010802027423号
京公网安备 11010802027423号