当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐Pot Mannich, Aza‐Wittig and Dehydrofluorinative Aromatization Reactions for Direct Synthesis of 2,3‐Disubstituted 4‐Aminoquinolines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-29 , DOI: 10.1002/adsc.202000734 Xiaofeng Zhang 1 , Xiaoming Ma 2 , Weiqi Qiu 3 , John Mark Awad 3 , Jason Evans 3 , Wei Zhang 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-29 , DOI: 10.1002/adsc.202000734 Xiaofeng Zhang 1 , Xiaoming Ma 2 , Weiqi Qiu 3 , John Mark Awad 3 , Jason Evans 3 , Wei Zhang 3
Affiliation
|
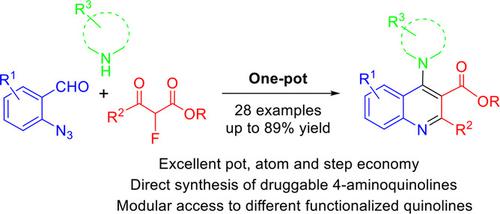
One‐pot synthesis of 2,3‐disubstituted 4‐aminoquinolines is developed through a three‐component reaction of 2‐azidobenzaldehydes, α‐fluoro‐β‐ketoesters and amines involving cascade Mannich, aza‐Wittig and dehydrofluorinative aromatization. The method is applicable to other functionalized quinolines. It is a pot, atom and step economy (PASE) synthesis of biologically interesting 4‐aminoquinolines relevant to hydroxychloroquine and chloroquine.
中文翻译:

直接合成2,3-二取代的4-氨基喹啉的一锅曼尼希,氮杂-维蒂希和脱氟化氢芳香化反应
通过2-叠氮苯甲醛,α-氟-β-酮酸酯和胺的三组分反应(包括级联曼尼希,氮杂-维蒂希和脱氟化氢芳构化)开发了2,3-二取代的4-氨基喹啉的一锅合成方法。该方法适用于其他官能化喹啉。这是一种与羟氯喹和氯喹相关的,具有生物学意义的4-氨基喹啉的锅,原子和步经济(PASE)合成。
更新日期:2020-12-08
中文翻译:

直接合成2,3-二取代的4-氨基喹啉的一锅曼尼希,氮杂-维蒂希和脱氟化氢芳香化反应
通过2-叠氮苯甲醛,α-氟-β-酮酸酯和胺的三组分反应(包括级联曼尼希,氮杂-维蒂希和脱氟化氢芳构化)开发了2,3-二取代的4-氨基喹啉的一锅合成方法。该方法适用于其他官能化喹啉。这是一种与羟氯喹和氯喹相关的,具有生物学意义的4-氨基喹啉的锅,原子和步经济(PASE)合成。









































 京公网安备 11010802027423号
京公网安备 11010802027423号