当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable CO/H2 Ratio on MoP Surfaces under Working Condition: a DFT based Thermodynamics Study
Surface Science ( IF 2.1 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.susc.2020.121738 Xinxin Tian , Jie Min , Tao Wang
Surface Science ( IF 2.1 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.susc.2020.121738 Xinxin Tian , Jie Min , Tao Wang
|
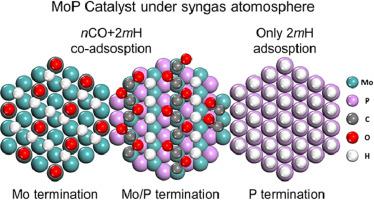
Abstract By performing a systematic DFT calculation and applying the atomistic thermodynamics analysis, the adsorption configurations, stable concentrations of nCO + mH2 co-adsorption on three representative MoP surfaces [(101), (001)-Mo and (001)-P] were investigated. Our results show that CO adsorbs much stronger than dissociative H2 on (101) and (001)-Mo surfaces but competitively with dissociative H2 on the (001)-P surface, and the hydrogen saturation coverage decreases with increasing CO pre-coverage. Ab initio atomistic thermodynamics analysis indicates the quite different CO and H2 co-adsorption manners on three surfaces under syngas atmosphere, i.e., at the equilibrium co-adsorption state, the Mo/P-terminated (101) surface and the Mo-terminated (001) surface have more versatile surface CO and H2 ratios, which are entirely different from that in the gas phase. However, the P-terminated (001) surface has only hydrogen adsorption at a wide range of conditions, which plays a role of hydrogen reservoir. Such investigations reveal that surface CO/H2 ratio could be altered by manipulating the pressures of the gas phase and temperatures, which would be beneficial to modify the syngas conversion reactivity as well as different product distributions on solid catalyst surfaces.
中文翻译:

工作条件下 MoP 表面上稳定的 CO/H2 比率:基于 DFT 的热力学研究
摘要 通过进行系统的 DFT 计算并应用原子热力学分析,三个代表性 MoP 表面 [(101)、(001)-Mo 和 (001)-P] 上的吸附构型、nCO + mH2 共吸附的稳定浓度为调查。我们的结果表明,CO 在 (101) 和 (001)-Mo 表面上吸附比解离 H2 强得多,但与 (001)-P 表面上的解离 H2 竞争,并且氢饱和覆盖率随着 CO 预覆盖率的增加而降低。从头算原子热力学分析表明,在合成气气氛下三个表面上CO和H2的共吸附方式完全不同,即在平衡共吸附状态下,Mo/P-封端(101)表面和Mo-封端(001)表面。 ) 表面具有更通用的表面 CO 和 H2 比率,这与气相中的完全不同。然而,P-封端的(001)表面在很宽的条件下只吸附氢,起到储氢的作用。此类研究表明,可以通过操纵气相压力和温度来改变表面 CO/H2 比,这将有利于改变合成气转化反应性以及固体催化剂表面上的不同产物分布。
更新日期:2021-01-01
中文翻译:

工作条件下 MoP 表面上稳定的 CO/H2 比率:基于 DFT 的热力学研究
摘要 通过进行系统的 DFT 计算并应用原子热力学分析,三个代表性 MoP 表面 [(101)、(001)-Mo 和 (001)-P] 上的吸附构型、nCO + mH2 共吸附的稳定浓度为调查。我们的结果表明,CO 在 (101) 和 (001)-Mo 表面上吸附比解离 H2 强得多,但与 (001)-P 表面上的解离 H2 竞争,并且氢饱和覆盖率随着 CO 预覆盖率的增加而降低。从头算原子热力学分析表明,在合成气气氛下三个表面上CO和H2的共吸附方式完全不同,即在平衡共吸附状态下,Mo/P-封端(101)表面和Mo-封端(001)表面。 ) 表面具有更通用的表面 CO 和 H2 比率,这与气相中的完全不同。然而,P-封端的(001)表面在很宽的条件下只吸附氢,起到储氢的作用。此类研究表明,可以通过操纵气相压力和温度来改变表面 CO/H2 比,这将有利于改变合成气转化反应性以及固体催化剂表面上的不同产物分布。











































 京公网安备 11010802027423号
京公网安备 11010802027423号