Leukemia Research ( IF 2.2 ) Pub Date : 2020-09-29 , DOI: 10.1016/j.leukres.2020.106458 Paul W. Manley , Louise Barys , Sandra W. Cowan-Jacob
|
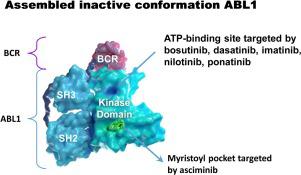
Asciminib is a potent, orally bioavailable, investigational drug that specifically and potently inhibits the tyrosine kinase activity of native ABL1, together with that of the chimeric BCR-ABL1 oncoprotein which causes chronic myeloid leukemia (CML). In contrast to ATP-competitive BCR-ABL1 kinase inhibitors employed to treat CML that target multiple kinases, asciminib binds to the myristate binding pocket on the kinase domains of ABL1 and BCR-ABL1. Hitherto no drugs have been developed whose mechanism of action involves interacting with myristate binding pockets on proteins, and analysis of the structures of such binding sites in proteins other than ABL1/BCR-ABL1 strongly suggest that asciminib will not bind to these with high affinity. Accordingly, the drug has no known safety liabilities resulting from any off-target activity, as illustrated by its specificity towards cells expressing BCR-ABL1 and lack of effects on non-kinase targets in biochemical screens. Because asciminib does not bind to the ATP-binding site it maintains substantial activity against kinase domain mutations that impart acquired drug resistance to ATP-competitive drugs. However, in vitro studies in cells have identified BCR-ABL1 mutations that reduce the anti-proliferative activity of asciminib, some of which are associated with clinical resistance towards the drug in patients. Here we review effects of asciminib on mutant forms of BCR-ABL1, analyse their sensitivity towards the drug from a structural perspective and affirm support for employing combinations with ATP-competitive inhibitors to impede the reactivation of BCR-ABL1 kinase activity in patients receiving monotherapy.
中文翻译:

作为一种肉豆蔻口袋结合的ABL抑制剂,asciminib(一种潜在的慢性粒细胞白血病治疗方法)的特异性及其与BCR-ABL1激酶突变体相互作用的分析
Asciminib是一种有效的,口服可生物利用的研究药物,可特异性和有效地抑制天然ABL1以及引起慢性粒细胞白血病(CML)的嵌合BCR-ABL1癌蛋白的酪氨酸激酶活性。与用于治疗靶向多种激酶的CML的ATP竞争性BCR-ABL1激酶抑制剂相反,asciminib与ABL1和BCR-ABL1激酶域上的肉豆蔻酸结合袋结合。迄今为止,尚未开发出其作用机理涉及与蛋白质上的肉豆蔻酸结合口袋相互作用的药物,并且对除ABL1 / BCR-ABL1以外的蛋白质中的此类结合位点的结构的分析强烈表明,asciminib将不会以高亲和力与它们结合。因此,该药物不存在因任何脱靶活动引起的已知安全性责任,如其对表达BCR-ABL1的细胞的特异性以及在生化筛选中对非激酶靶点缺乏影响所证明。因为asciminib不与ATP结合位点结合,所以它保持了对激酶结构域突变的实质活性,该突变使赋予ATP竞争性药物耐药性。然而,在细胞中进行的体外研究发现,BCR-ABL1突变可降低asciminib的抗增殖活性,其中一些与患者对该药的临床耐药性有关。在这里,我们审查了asciminib对BCR-ABL1突变形式的影响,从结构角度分析了其对药物的敏感性,并确认支持与ATP竞争性抑制剂联合使用以阻止接受单一疗法的患者重新激活BCR-ABL1激酶活性。























































 京公网安备 11010802027423号
京公网安备 11010802027423号