Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-09-30 , DOI: 10.1016/j.apsb.2020.09.015 Jianfeng Wang 1 , Xiaomao Yin 1 , Wei He 2 , Wei Xue 1 , Jin Zhang 1 , Yiran Huang 1
|
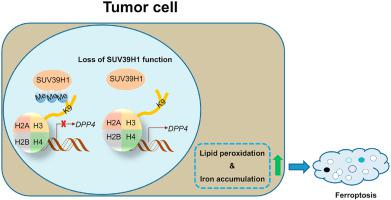
Clear cell renal cell carcinoma (ccRCC) is a common kidney malignancy characterized by a poor prognosis. Suppressor of variegation 3–9 homolog 1 (SUV39H1), which encodes a histone H3 lysine 9 methyltransferase, has been reported to act as an oncogene in many cancers. However, it is unclear whether SUV39H1 is involved in ccRCC. Here, we report that SUV39H1 expression is frequently upregulated in ccRCC tumors and is significantly correlated with ccRCC progression. SUV39H1 expression level is an independent risk factor for cancer prognosis, and integration with several known prognostic factors predicted ccRCC patient prognosis with improved accuracy than the conventional SSIGN (stage, size, grade and necrosis) prognostic model. Mechanistically, we discovered that siRNA knockdown or pharmacological inhibition of SUV39H1 induced iron accumulation and lipid peroxidation, leading to ferroptosis that disrupted ccRCC cell growth in vitro and in vivo. We also show that SUV39H1 deficiency modulated the H3K9me3 status of the DPP4 (dipeptidyl-peptidase-4) gene promoter, resulting in upregulation of its expression that contributes to ferroptosis. Taken together, our findings provide the mechanistic insight into SUV39H1-dependent epigenetic control of ccRCC tumor growth and indicate that SUV39H1 may serve as a potential therapeutic target for ccRCC treatment.
中文翻译:

SUV39H1缺陷通过诱导铁死亡抑制透明细胞肾细胞癌的生长
透明细胞肾细胞癌(ccRCC)是一种常见的肾脏恶性肿瘤,其特点是预后不良。据报道,杂色抑制因子 3-9 同源物 1 ( SUV39H1 ) 编码组蛋白 H3 赖氨酸 9 甲基转移酶,在许多癌症中充当癌基因。然而,尚不清楚SUV39H1是否参与ccRCC。在这里,我们报告SUV39H1表达在 ccRCC 肿瘤中频繁上调,并且与 ccRCC 进展显着相关。 SUV39H1表达水平是癌症预后的独立危险因素,与几个已知的预后因素相结合预测ccRCC患者的预后,其准确性比传统的SSIGN(分期、大小、分级和坏死)预后模型更高。从机制上讲,我们发现SUV39H1的 siRNA 敲低或药理学抑制会诱导铁积累和脂质过氧化,导致铁死亡,从而破坏体外和体内ccRCC 细胞的生长。我们还表明, SUV39H1缺陷调节DPP4 (二肽基肽酶 4)基因启动子的 H3K9me3 状态,导致其表达上调,从而导致铁死亡。总而言之,我们的研究结果为 ccRCC 肿瘤生长的SUV39H1依赖性表观遗传控制提供了机制见解,并表明SUV39H1可能作为 ccRCC 治疗的潜在治疗靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号