当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium‐Catalysed Reductive Deoxygenation of Ketones with Formic Acid as Traceless Hydride Donor
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-28 , DOI: 10.1002/adsc.202000821 Zhiheng Yang 1 , Xueya Zhu 1 , Shiyi Yang 1 , Cheng Weiyan 1 , Xiaojian Zhang 1 , Zhanhui Yang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-28 , DOI: 10.1002/adsc.202000821 Zhiheng Yang 1 , Xueya Zhu 1 , Shiyi Yang 1 , Cheng Weiyan 1 , Xiaojian Zhang 1 , Zhanhui Yang 1
Affiliation
|
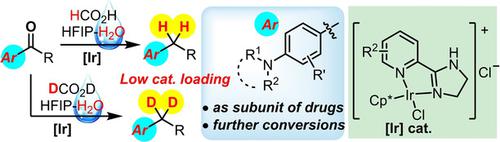
An iridium‐catalysed deoxygenation of ketones and aldehydes is achieved, with formic acid as hydride donor and water as co‐solvent. At low catalyst loading, a number of 4‐(N,N‐disubstituted amino) aryl ketones are readily deoxygenated in excellent yields and chemoselectivity. Numerous functional groups, especially phenolic and alcoholic hydroxyls, secondary amine, carboxylic acid, and alkyl chloride, are well tolerable. Geminally dideuterated alkanes are obtained with up to 90% D incorporation, when DCO2D and D2O are used in place of their hydrogenative counterparts. The activating 4‐(N,N‐disubstituted amino)aryl groups have been demonstrated to undergo a variety of useful transformations. The deoxygenative deuterations have been used to prepare a deuterated drug molecule Chlorambucil‐4,4‐d2.
中文翻译:

铱作为甲酸的痕量氢化物供体在铱上催化酮的还原性脱氧
以甲酸为氢化物供体,水为助溶剂,实现了铱催化的酮和醛的脱氧。在低催化剂负载下,许多4-(N,N-二取代氨基)芳基酮易于脱氧,具有出色的收率和化学选择性。许多官能团,尤其是酚羟基和醇羟基,仲胺,羧酸和烷基氯,都具有良好的耐受性。当使用DCO 2 D和D 2 O代替它们的氢化对应物时,可获得双歧化的双链烷烃达90%。激活4‐(N,N已证明二取代氨基)芳基会经历各种有用的转化。脱氧氘已用于制备氘代药物分子Chlorambucil-4,4- d 2。
更新日期:2020-09-28
中文翻译:

铱作为甲酸的痕量氢化物供体在铱上催化酮的还原性脱氧
以甲酸为氢化物供体,水为助溶剂,实现了铱催化的酮和醛的脱氧。在低催化剂负载下,许多4-(N,N-二取代氨基)芳基酮易于脱氧,具有出色的收率和化学选择性。许多官能团,尤其是酚羟基和醇羟基,仲胺,羧酸和烷基氯,都具有良好的耐受性。当使用DCO 2 D和D 2 O代替它们的氢化对应物时,可获得双歧化的双链烷烃达90%。激活4‐(N,N已证明二取代氨基)芳基会经历各种有用的转化。脱氧氘已用于制备氘代药物分子Chlorambucil-4,4- d 2。









































 京公网安备 11010802027423号
京公网安备 11010802027423号