当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Type I interferon regulates cytokine‐delayed neutrophil apoptosis, reactive oxygen species production and chemokine expression
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-09-29 , DOI: 10.1111/cei.13525 L Glennon-Alty 1, 2 , R J Moots 3 , S W Edwards 4 , H L Wright 1
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-09-29 , DOI: 10.1111/cei.13525 L Glennon-Alty 1, 2 , R J Moots 3 , S W Edwards 4 , H L Wright 1
Affiliation
|
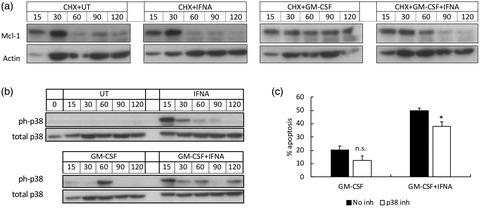
Interferons (IFNs) are key regulators of a number of inflammatory conditions in which neutrophils play an important role in pathology, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), where type I IFNs are implicated in disease pathology. However, IFNs are usually generated in vivo together with other cytokines that also have immunoregulatory functions, but such interactions are poorly defined experimentally. We measured the effects of type I (IFN‐α) IFN, elevated in both RA and SLE, on the functions of healthy neutrophils incubated in vitro in the absence and presence of proinflammatory cytokines typically elevated in inflammatory diseases [tumour necrosis factor (TNF‐α), granulocyte–macrophage colony‐stimulating factor (GM‐CSF)]. IFN‐α alone had no effect on neutrophil apoptosis; however, it abrogated the anti‐apoptotic effect of GM‐CSF (18 h, P < 0·01). The enhanced stability of the anti‐apoptotic protein myeloid cell leukaemia 1 (Mcl‐1) and delayed activation of caspase activation normally regulated by GM‐CSF were blocked by IFN‐α: this effect was mediated, in part, by activation of p38 mitogen‐activated protein kinase (MAPK). IFN‐α alone also primed reactive oxygen species (ROS) production and maintained the transient priming effect of TNF‐α for up to 4 h: it also down‐regulated GM‐CSF‐ and TNF‐α‐activated expression of chemokine (C‐X‐C motif) ligand (CXCL)1, CXCL2, CXCL3, CXCL8, CCL3 and CCL4 but, in contrast, increased the expression of CXCL10. These novel data identify complex regulatory signalling networks in which type I IFNs profoundly alter the response of neutrophils to inflammatory cytokines. This is likely to have important consequences in vivo and may explain the complexity and heterogeneity of inflammatory diseases such as RA, in which multiple cytokine cascades have been activated.
中文翻译:

I型干扰素调节细胞因子延迟的中性粒细胞凋亡、活性氧产生和趋化因子表达
干扰素 (IFN) 是许多炎症状况的关键调节因子,其中中性粒细胞在病理学中发挥重要作用,例如类风湿性关节炎 (RA) 和系统性红斑狼疮 (SLE),其中 I 型干扰素与疾病病理学有关。然而,干扰素通常在体内与其他也具有免疫调节功能的细胞因子一起产生,但这种相互作用在实验上尚不清楚。我们测量了在 RA 和 SLE 中升高的 I 型 (IFN-α) IFN 对体外培养的健康中性粒细胞功能的影响在不存在和存在促炎细胞因子的情况下,炎症性疾病中通常会升高 [肿瘤坏死因子 (TNF-α)、粒细胞 - 巨噬细胞集落刺激因子 (GM-CSF)]。单独使用 IFN-α 对中性粒细胞凋亡没有影响;然而,它消除了 GM-CSF 的抗凋亡作用(18 小时,P < 0·01)。抗凋亡蛋白骨髓细胞白血病 1 (Mcl-1) 的稳定性增强和通常由 GM-CSF 调节的半胱天冬酶激活延迟激活被 IFN-α 阻断:这种作用部分是通过激活 p38 丝裂原介导的活化蛋白激酶(MAPK)。单独的 IFN-α 也引发了活性氧 (ROS) 的产生,并将 TNF-α 的瞬时启动作用维持了长达 4 小时:它还下调了 GM-CSF- 和 TNF-α 激活的趋化因子 (C- X-C 基序)配体 (CXCL)1、CXCL2、CXCL3、CXCL8、CCL3 和 CCL4,但相反,增加了 CXCL10 的表达。这些新数据确定了复杂的调节信号网络,其中 I 型干扰素深刻地改变了中性粒细胞对炎性细胞因子的反应。这很可能在体内产生重要的后果并且可以解释炎症性疾病如 RA 的复杂性和异质性,其中多个细胞因子级联反应已被激活。
更新日期:2020-09-29
中文翻译:

I型干扰素调节细胞因子延迟的中性粒细胞凋亡、活性氧产生和趋化因子表达
干扰素 (IFN) 是许多炎症状况的关键调节因子,其中中性粒细胞在病理学中发挥重要作用,例如类风湿性关节炎 (RA) 和系统性红斑狼疮 (SLE),其中 I 型干扰素与疾病病理学有关。然而,干扰素通常在体内与其他也具有免疫调节功能的细胞因子一起产生,但这种相互作用在实验上尚不清楚。我们测量了在 RA 和 SLE 中升高的 I 型 (IFN-α) IFN 对体外培养的健康中性粒细胞功能的影响在不存在和存在促炎细胞因子的情况下,炎症性疾病中通常会升高 [肿瘤坏死因子 (TNF-α)、粒细胞 - 巨噬细胞集落刺激因子 (GM-CSF)]。单独使用 IFN-α 对中性粒细胞凋亡没有影响;然而,它消除了 GM-CSF 的抗凋亡作用(18 小时,P < 0·01)。抗凋亡蛋白骨髓细胞白血病 1 (Mcl-1) 的稳定性增强和通常由 GM-CSF 调节的半胱天冬酶激活延迟激活被 IFN-α 阻断:这种作用部分是通过激活 p38 丝裂原介导的活化蛋白激酶(MAPK)。单独的 IFN-α 也引发了活性氧 (ROS) 的产生,并将 TNF-α 的瞬时启动作用维持了长达 4 小时:它还下调了 GM-CSF- 和 TNF-α 激活的趋化因子 (C- X-C 基序)配体 (CXCL)1、CXCL2、CXCL3、CXCL8、CCL3 和 CCL4,但相反,增加了 CXCL10 的表达。这些新数据确定了复杂的调节信号网络,其中 I 型干扰素深刻地改变了中性粒细胞对炎性细胞因子的反应。这很可能在体内产生重要的后果并且可以解释炎症性疾病如 RA 的复杂性和异质性,其中多个细胞因子级联反应已被激活。











































 京公网安备 11010802027423号
京公网安备 11010802027423号