当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aligned human cardiac syncytium for in vitro analysis of electrical, structural, and mechanical readouts
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-09-29 , DOI: 10.1002/bit.27582 B N Napiwocki 1, 2 , D Lang 3 , A Stempien 1, 2 , J Zhang 3 , R Vaidyanathan 3 , J C Makielski 3 , L L Eckhardt 3 , A V Glukhov 3 , T J Kamp 3, 4 , W C Crone 1, 2, 5
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-09-29 , DOI: 10.1002/bit.27582 B N Napiwocki 1, 2 , D Lang 3 , A Stempien 1, 2 , J Zhang 3 , R Vaidyanathan 3 , J C Makielski 3 , L L Eckhardt 3 , A V Glukhov 3 , T J Kamp 3, 4 , W C Crone 1, 2, 5
Affiliation
|
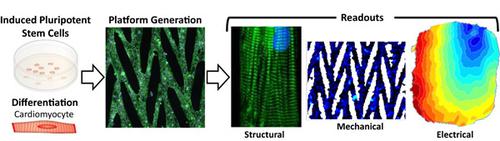
Human pluripotent stem cell‐derived cardiomyocytes (hPSC‐CMs) have emerged as an exciting new tool for cardiac research and can serve as a preclinical platform for drug development and disease modeling studies. However, these aspirations are limited by current culture methods in which hPSC‐CMs resemble fetal human cardiomyocytes in terms of structure and function. Herein we provide a novel in vitro platform that includes patterned extracellular matrix with physiological substrate stiffness and is amenable to both mechanical and electrical analysis. Micropatterned lanes promote the cellular and myofibril alignment of hPSC‐CMs while the addition of micropatterned bridges enable formation of a functional cardiac syncytium that beats synchronously over a large two‐dimensional area. We investigated the electrophysiological properties of the patterned cardiac constructs and showed they have anisotropic electrical impulse propagation, as occurs in the native myocardium, with speeds 2x faster in the primary direction of the pattern as compared to the transverse direction. Lastly, we interrogated the mechanical function of the pattern constructs and demonstrated the utility of this platform in recording the strength of cardiomyocyte contractions. This biomimetic platform with electrical and mechanical readout capabilities will enable the study of cardiac disease and the influence of pharmaceuticals and toxins on cardiomyocyte function. The platform also holds potential for high throughput evaluation of drug safety and efficacy, thus furthering our understanding of cardiovascular disease and increasing the translational use of hPSC‐CMs.
中文翻译:

对齐的人类心脏合胞体,用于体外分析电气、结构和机械读数
人类多能干细胞衍生的心肌细胞 (hPSC-CM) 已成为一种令人兴奋的心脏研究新工具,并可作为药物开发和疾病建模研究的临床前平台。然而,这些愿望受到当前培养方法的限制,其中 hPSC-CM 在结构和功能方面类似于人类胎儿心肌细胞。在这里,我们提供了一个新的体外平台,其中包括具有生理基底刚度的图案化细胞外基质,并且适用于机械和电分析。微图案通道促进 hPSC-CM 的细胞和肌原纤维排列,而微图案桥的添加能够形成功能性心脏合胞体,在大的二维区域内同步跳动。我们研究了图案化心脏结构的电生理学特性,并表明它们具有各向异性的电脉冲传播,正如在天然心肌中发生的那样,与横向相比,图案主要方向上的速度快 2 倍。最后,我们询问了模式结构的机械功能,并证明了该平台在记录心肌细胞收缩强度方面的效用。这种具有电子和机械读出功能的仿生平台将能够研究心脏病以及药物和毒素对心肌细胞功能的影响。该平台还具有对药物安全性和有效性进行高通量评估的潜力,
更新日期:2020-09-29
中文翻译:

对齐的人类心脏合胞体,用于体外分析电气、结构和机械读数
人类多能干细胞衍生的心肌细胞 (hPSC-CM) 已成为一种令人兴奋的心脏研究新工具,并可作为药物开发和疾病建模研究的临床前平台。然而,这些愿望受到当前培养方法的限制,其中 hPSC-CM 在结构和功能方面类似于人类胎儿心肌细胞。在这里,我们提供了一个新的体外平台,其中包括具有生理基底刚度的图案化细胞外基质,并且适用于机械和电分析。微图案通道促进 hPSC-CM 的细胞和肌原纤维排列,而微图案桥的添加能够形成功能性心脏合胞体,在大的二维区域内同步跳动。我们研究了图案化心脏结构的电生理学特性,并表明它们具有各向异性的电脉冲传播,正如在天然心肌中发生的那样,与横向相比,图案主要方向上的速度快 2 倍。最后,我们询问了模式结构的机械功能,并证明了该平台在记录心肌细胞收缩强度方面的效用。这种具有电子和机械读出功能的仿生平台将能够研究心脏病以及药物和毒素对心肌细胞功能的影响。该平台还具有对药物安全性和有效性进行高通量评估的潜力,











































 京公网安备 11010802027423号
京公网安备 11010802027423号