Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2020-07-27 , DOI: 10.1016/j.gresc.2020.05.005 Haifeng Wang , Shuangxi Gu , Qiongjiao Yan , Li Ding , Fen-Er Chen
|
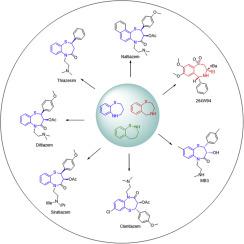
Chiral benzothiazepines constitute the core structures of many foremost pharmaceuticals with diverse biological activities endowed by their unique scaffolds, which poses a great challenge to organic chemists and pharmaceutical researchers. This review provides a concise overview for the asymmetric synthesis of chiral benzothiazepine derivatives, focusing on advances in asymmetric catalysis, including metal catalysis, small-molecule organocatalysis and enzymatic catalysis. The catalytic asymmetric reactions, involving asymmetric epoxidation, reduction, dihydroxylation, hydrogenation, aldol reaction and other sulfa-Michael addition, have emerged as powerful strategies for the rapid construction of chiral benzothiazepine through single or multistep reactions. The booming asymmetric synthetic methodology affords us instructive clues for the highly efficient preparation of chiral benzothiazepines, facilitating their large-scale preparation and diversity-oriented synthesis.
中文翻译:

手性苯并硫氮杂s的合成策略中的不对称催化
手性苯并硫氮杂constitute类化合物是许多最重要药物的核心结构,它们具有独特的支架,具有多种生物活性,这对有机化学家和药物研究人员构成了巨大挑战。这篇综述简要概述了手性苯并硫氮杂the衍生物的不对称合成,重点是不对称催化的进展,包括金属催化,小分子有机催化和酶催化。催化不对称反应,包括不对称环氧化,还原,二羟基化,氢化,羟醛反应和其他磺胺-迈克尔加成反应,已成为通过单步或多步反应快速构建手性苯并硫氮杂pine的有力策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号