当前位置:
X-MOL 学术
›
Green Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of -tertiary allylsilanes by palladium-catalyzed hydrosilylation of 1,1-disubstituted allenes
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2020-12-1 , DOI: 10.1016/j.gresc.2020.08.003 Kaidi Li , Ming Nie , Wenjun Tang
Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2020-12-1 , DOI: 10.1016/j.gresc.2020.08.003 Kaidi Li , Ming Nie , Wenjun Tang
|
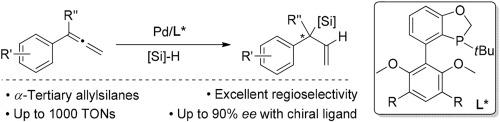
We herein report an effective hydrosilylation of 1,1-disubstituted allenes with a palladium catalyst modified by a monophosphorus ligand, leading to a series of ¦Á-tertiary allylsilanes in high yields and exceptional regiose-lectivities. The employment of BI-DIME is essential for the high reactivities and selectivities. A chiral ¦Á-tertiary allylsilane is formed in excellent ee for the first time by employing a chiral monophosphorus ligand.
中文翻译:

钯催化的1,1-二取代的烯丙基硅氢加成反应合成叔丁基硅烷
我们在本文中报道了用单磷配体改性的钯催化剂对1,1-二取代的烯进行有效的硅氢加成反应,从而以高收率和优异的区域选择性产生了一系列α-叔烯丙基硅烷。BI-DIME的使用对于高反应活性和选择性至关重要。通过使用手性单磷配体,首次在优异的ee中形成了手性α-叔烯丙基硅烷。
更新日期:2020-12-28
中文翻译:

钯催化的1,1-二取代的烯丙基硅氢加成反应合成叔丁基硅烷
我们在本文中报道了用单磷配体改性的钯催化剂对1,1-二取代的烯进行有效的硅氢加成反应,从而以高收率和优异的区域选择性产生了一系列α-叔烯丙基硅烷。BI-DIME的使用对于高反应活性和选择性至关重要。通过使用手性单磷配体,首次在优异的ee中形成了手性α-叔烯丙基硅烷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号