Water Research ( IF 11.4 ) Pub Date : 2020-09-29 , DOI: 10.1016/j.watres.2020.116481 Dandan Rao , Jie Chen , Hongyu Dong , Junlian Qiao , Baoxue Zhou , Yuankui Sun , Xiaohong Guan
|
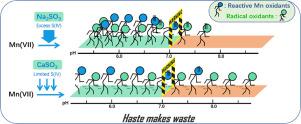
Although permanganate activation by sodium sulfite (Mn(VII)/Na2SO3) has shown great potential for rapid abatement of organic contaminants, the limited reactivity under alkaline conditions and undesirable Mn residual may prevent its widespread application. To solve these challenges, calcium sulfite (CaSO3) was employed as a slow-release source of SO32−/HSO3− (S(IV)) to activate Mn(VII) in this study. It was found that the application of CaSO3 solid could extend the effective working pH range of Mn(VII)/S(IV) from ≤7.0 to ≤9.0. Moreover, due to the enhanced precipitation of MnO2 with the presence of Ca2+, very low Mn residual (<0.05 mg/L) was achieved in Mn(VII)/CaSO3 system. Mn(VII)/CaSO3 system is a unique two-stage oxidation process in terms of reaction kinetics and reactive oxidants. Specifically, Mn(VII) was rapidly consumed and reactive Mn intermediates (e.g., Mn(VI), Mn(V)), SO4•−, and HO• were produced in the first stage. However, the second stage was governed by the interaction between MnO2 and S(IV), with SO4•− and HO• serving as the dominant reactive oxidants. Taking advantage of an automatic titrator, excess S(IV) was found to greatly quench the generated radicals, whereas it did not cause a significant consumption of reactive Mn species. All these results improved our understanding of the Mn(VII)/S(IV) process and could thus facilitate its application.
中文翻译:

与环境有关的条件下Mn(VII)/ CaSO 3增强有机污染物的氧化:性能和机理
尽管通过亚硫酸钠(Mn(VII)/ Na 2 SO 3)活化高锰酸盐显示出迅速消除有机污染物的巨大潜力,但在碱性条件下有限的反应性和不希望的Mn残留物可能会阻止其广泛应用。为了解决这些难题,亚硫酸钙(的CaSO 3)作为SO的缓释源3 2- / HSO 3 -(S(IV)),以在该研究中激活的Mn(VII)。发现使用CaSO 3固体可以将Mn(VII)/ S(IV)的有效工作pH范围从≤7.0扩展到≤9.0。此外,由于存在Ca 2+时MnO 2的沉淀增加,在Mn(VII)/ CaSO 3体系中实现了极低的Mn残留(<0.05 mg / L)。就反应动力学和反应性氧化剂而言,Mn(VII)/ CaSO 3系统是独特的两阶段氧化过程。具体地,Mn(VII)被快速消耗,并且在第一阶段中产生反应性Mn中间体(例如,Mn(VI),Mn(V)),SO 4 •-和HO •。但是,第二阶段是由MnO 2和S(IV)与SO 4 •−和HO •的相互作用决定的。作为主要的反应性氧化剂。利用自动滴定仪,发现过量的S(IV)可以极大地淬灭生成的自由基,而不会引起活性Mn种类的大量消耗。所有这些结果提高了我们对Mn(VII)/ S(IV)工艺的理解,因此可以促进其应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号