Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-09-29 , DOI: 10.1016/j.jinorgbio.2020.111262 Samantha M Powell 1 , Leonard M Thomas 1 , George B Richter-Addo 1
|
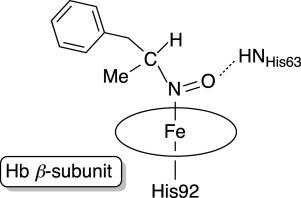
Amphetamine-based (Amph) drugs are metabolized in humans to their hydroxylamine (AmphNHOH) and nitroso (AmphNO) derivatives. The latter metabolites are known to bind to the Fe centers of cytochrome P450 and other heme enzymes to inhibit their activities. Although these AmphNHOH/AmphNO metabolites are present in vivo, their interactions with the blood protein hemoglobin (Hb) and the muscle protein (Mb) have been largely discounted due to a perception that the relatively small heme active sites of Hb and Mb will not be able to accommodate the large AmphNO group. We report the 2.15 Å resolution X-ray crystal structure of the AmphNO adduct of adult human hemoglobin as the Hb [α-FeIII(H2O)][β-FeII(AmphNO)] derivative. We show that the binding of AmphNO to the β subunit is enabled by an E helix movement and stabilization of ligand binding by H-bonding with the distal His63 residue. We also observe an AmphNHOH group in the Xe2 pocket in close proximity to the α heme site in this derivative. Additionally, UV–vis spectroscopy was used to characterize this and related wt and mutant Mb adducts. Importantly, our X-ray crystal structure of this Hb-nitrosoamphetamine complex represents the first crystal structure of a wild-type heme protein adduct of any amphetamine metabolite. Our results provide a framework for further studies of AmphNHOH/AmphNO interactions with Hb and Mb as viable processes that potentially contribute to the overall biological inorganic chemistry of amphetamine drugs.
中文翻译:

亚硝基苯丙胺代谢物位于人血红蛋白的活性部位:光谱学和晶体结构
苯丙胺类 (Amph) 药物在人体中代谢为羟胺 (AmphNHOH) 和亚硝基 (AmphNO) 衍生物。已知后者的代谢物与细胞色素 P450 和其他血红素酶的 Fe 中心结合以抑制它们的活性。尽管这些 AmphNHOH/AmphNO 代谢物存在于体内,但它们与血液蛋白血红蛋白 (Hb) 和肌肉蛋白 (Mb) 的相互作用在很大程度上被低估了,因为人们认为 Hb 和 Mb 的相对较小的血红素活性位点不会能够容纳大型 AmphNO 组。我们将成人血红蛋白的 AmphNO 加合物的 2.15 Å 分辨率 X 射线晶体结构报告为 Hb [ α -Fe III (H 2 O)][ β -Fe II(AmphNO)] 衍生物。我们表明,AmphNO 与β亚基的结合是通过 E 螺旋运动和配体结合的稳定化实现的,即通过与远端 His63 残基的 H 键合。我们还在靠近α的 Xe2 口袋中观察到 AmphNHOH 基团血红素站点在这个衍生物。此外,紫外-可见光谱用于表征这种以及相关的 wt 和突变 Mb 加合物。重要的是,我们的这种 Hb-亚硝基苯丙胺复合物的 X 射线晶体结构代表了任何苯丙胺代谢物的野生型血红素蛋白加合物的第一个晶体结构。我们的结果为进一步研究 AmphNHOH/AmphNO 与 Hb 和 Mb 的相互作用提供了框架,这些相互作用可能有助于苯丙胺药物的整体生物无机化学。










































 京公网安备 11010802027423号
京公网安备 11010802027423号