当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 6-substituted indoloquinazolinones from arynes and 2-acyl-4-quinazolinones: a transition-metal free C–N and C–C bond formation strategy
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-09-28 , DOI: 10.1039/d0nj04395h Abhilash Sharma 1, 2, 3, 4, 5 , Pranjal Gogoi 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-09-28 , DOI: 10.1039/d0nj04395h Abhilash Sharma 1, 2, 3, 4, 5 , Pranjal Gogoi 1, 2, 3, 4, 5
Affiliation
|
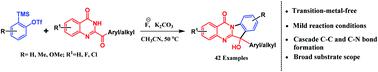
A versatile transition-metal free synthetic strategy has been developed for the direct synthesis of 6-substituted indoloquinazolinones from 2-acyl-4-quinazolinones and aryne precursors under mild conditions. This cascade strategy proceeds via successive C–N and C–C bond formation in a single reaction vessel by simple treatment with CsF and K2CO3. A diverse range of 6-substituted indoloquinazolinones were synthesized in good yields with excellent functional group tolerance.
中文翻译:

由芳烃和2-酰基-4-喹唑啉酮合成6-取代的吲哚喹唑啉酮:无过渡金属的CN和CC键形成策略
已经开发了一种通用的无过渡金属的合成策略,用于在温和条件下由2-酰基-4-喹唑啉酮和芳烃前体直接合成6-取代的吲哚喹唑啉酮。这种级联策略是通过在单个反应容器中用CsF和K 2 CO 3进行简单处理而连续形成C–N和C–C键来进行的。以良好的产率和优异的官能团耐受性合成了多种6-取代的吲哚并喹唑啉酮。
更新日期:2020-10-13
中文翻译:

由芳烃和2-酰基-4-喹唑啉酮合成6-取代的吲哚喹唑啉酮:无过渡金属的CN和CC键形成策略
已经开发了一种通用的无过渡金属的合成策略,用于在温和条件下由2-酰基-4-喹唑啉酮和芳烃前体直接合成6-取代的吲哚喹唑啉酮。这种级联策略是通过在单个反应容器中用CsF和K 2 CO 3进行简单处理而连续形成C–N和C–C键来进行的。以良好的产率和优异的官能团耐受性合成了多种6-取代的吲哚并喹唑啉酮。









































 京公网安备 11010802027423号
京公网安备 11010802027423号