当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Toxic Metamorphosis—How Changes from Lysosomal to Cytosolic pH Modify the Alpha-Synuclein Aggregation Pattern
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.biomac.0c00629 Bisher Eymsh 1, 2 , Alice Drobny 1 , Timon R Heyn 3 , Wei Xiang 4 , Ralph Lucius 2 , Karin Schwarz 3 , Julia K Keppler 3, 5 , Friederike Zunke 1 , Philipp Arnold 2, 6
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.biomac.0c00629 Bisher Eymsh 1, 2 , Alice Drobny 1 , Timon R Heyn 3 , Wei Xiang 4 , Ralph Lucius 2 , Karin Schwarz 3 , Julia K Keppler 3, 5 , Friederike Zunke 1 , Philipp Arnold 2, 6
Affiliation
|
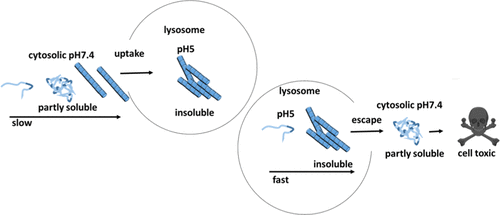
Alpha-synuclein (aSyn) is a cytosolic, aggregation-prone protein that is associated with neurodegenerative disorders like Parkinson’s disease. Interestingly, the protein can appear in different conformations, including monomeric and oligomeric forms as well as amyloid fibrils. Its individual structural constituents seem to be dependent on various factors and the composition of the respective cellular surroundings. Although under physiological conditions, most aSyn is found in the cytosol and synapses of neurons, aSyn can also be found in lysosomal compartments, where it gets degraded. We here compare the assembly speed, morphology, folding state, and spreading of aSyn at cytosolic pH (pH 7.4) and lysosomal pH (pH 5) using Thioflavin T, transmission electron microscopy, circular dichroism, and Fourier transform infrared spectroscopy. Interestingly, we found substantial differences between aSyn aggregation under neutral and acidic pH conditions, like those present in cytosolic and lysosomal cellular compartments. Also, lysosomal aSyn enriched from an aSyn-overexpressing cell line was able to seed aggregation in a concentration-dependent manner. Moreover, we observed that aSyn aggregates formed under in vitro lysosomal pH (pH 5) conditions were not stable at neutral pH and collapsed into partly soluble aggregates with changed structural characteristics. Our findings have meaningful implications in intracellular toxicity events as well as in lysis procedures for molecular and structural characterization of intracellular aSyn conformers.
中文翻译:

毒性变态—从溶酶体到胞浆pH的变化如何改变α-突触核蛋白的聚集模式
α-突触核蛋白(aSyn)是一种胞质,易于聚集的蛋白质,与帕金森氏病等神经退行性疾病有关。有趣的是,蛋白质可以以不同的构象出现,包括单体和寡聚形式以及淀粉样原纤维。它的各个结构成分似乎取决于各种因素以及各个细胞周围环境的组成。尽管在生理条件下,大多数aSyn存在于神经元的胞质溶胶和突触中,但aSyn也存在于溶酶体区室中,并在那里被降解。我们在这里使用硫黄素T,透射电镜,圆二色性和傅立叶变换红外光谱法比较了胞浆pH(pH 7.4)和溶酶体pH(pH 5)时aSyn的组装速度,形态,折叠状态和扩散。有趣的是 我们发现在中性和酸性pH条件下aSyn聚集之间存在实质性差异,例如在胞质和溶酶体细胞区室中存在的聚集。同样,从过表达aSyn的细胞系富集的溶酶体aSyn能够以浓度依赖的方式播种聚集。此外,我们观察到在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并塌陷成结构特征改变的部分可溶性聚集体。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。像胞浆和溶酶体细胞区隔中的那些 同样,从过表达aSyn的细胞系富集的溶酶体aSyn能够以浓度依赖的方式播种聚集。此外,我们观察到在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并塌陷成结构特征改变的部分可溶性聚集体。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。像胞浆和溶酶体细胞区隔中的那些 同样,从过表达aSyn的细胞系富集的溶酶体aSyn能够以浓度依赖的方式播种聚集。此外,我们观察到在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并塌陷成结构特征改变的部分可溶性聚集体。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。我们观察到,在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并坍塌为部分易溶的聚集体,结构特征发生了变化。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。我们观察到,在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并坍塌为部分易溶的聚集体,结构特征发生了变化。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。
更新日期:2020-09-28
中文翻译:

毒性变态—从溶酶体到胞浆pH的变化如何改变α-突触核蛋白的聚集模式
α-突触核蛋白(aSyn)是一种胞质,易于聚集的蛋白质,与帕金森氏病等神经退行性疾病有关。有趣的是,蛋白质可以以不同的构象出现,包括单体和寡聚形式以及淀粉样原纤维。它的各个结构成分似乎取决于各种因素以及各个细胞周围环境的组成。尽管在生理条件下,大多数aSyn存在于神经元的胞质溶胶和突触中,但aSyn也存在于溶酶体区室中,并在那里被降解。我们在这里使用硫黄素T,透射电镜,圆二色性和傅立叶变换红外光谱法比较了胞浆pH(pH 7.4)和溶酶体pH(pH 5)时aSyn的组装速度,形态,折叠状态和扩散。有趣的是 我们发现在中性和酸性pH条件下aSyn聚集之间存在实质性差异,例如在胞质和溶酶体细胞区室中存在的聚集。同样,从过表达aSyn的细胞系富集的溶酶体aSyn能够以浓度依赖的方式播种聚集。此外,我们观察到在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并塌陷成结构特征改变的部分可溶性聚集体。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。像胞浆和溶酶体细胞区隔中的那些 同样,从过表达aSyn的细胞系富集的溶酶体aSyn能够以浓度依赖的方式播种聚集。此外,我们观察到在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并塌陷成结构特征改变的部分可溶性聚集体。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。像胞浆和溶酶体细胞区隔中的那些 同样,从过表达aSyn的细胞系富集的溶酶体aSyn能够以浓度依赖的方式播种聚集。此外,我们观察到在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并塌陷成结构特征改变的部分可溶性聚集体。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。我们观察到,在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并坍塌为部分易溶的聚集体,结构特征发生了变化。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。我们观察到,在体外溶酶体pH(pH 5)条件下形成的aSyn聚集体在中性pH处不稳定,并坍塌为部分易溶的聚集体,结构特征发生了变化。我们的发现对细胞内毒性事件以及细胞内aSyn构象分子和结构表征的裂解程序具有有意义的意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号