当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Internal Oligoguanidinium Transporter: Mercury-Free Scalable Synthesis, Improvement of Cellular Localization, Endosomal Escape, Mitochondrial Localization, and Conjugation with Antisense Morpholino for NANOG Inhibition to Induce Chemosensitization of Taxol in MCF-7 Cells
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.bioconjchem.0c00444 Jayanta Kundu 1 , Priyanjalee Banerjee 1 , Chandra Bose 1 , Ujjal Das 1 , Ujjwal Ghosh 1 , Surajit Sinha 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-09-28 , DOI: 10.1021/acs.bioconjchem.0c00444 Jayanta Kundu 1 , Priyanjalee Banerjee 1 , Chandra Bose 1 , Ujjal Das 1 , Ujjwal Ghosh 1 , Surajit Sinha 1
Affiliation
|
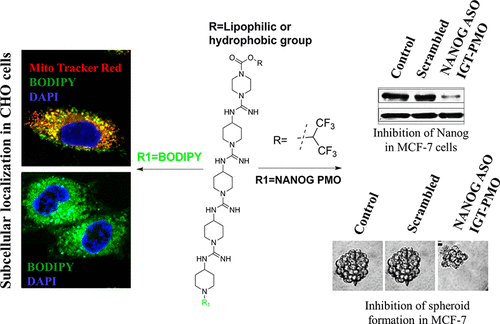
A nontoxic delivery vehicle is essential for the therapeutic applications of antisense phosphorodiamidate morpholino oligonucleotides (PMOs). Though guanidinium-rich or arginine-rich cellular transporter conjugated Vivo-PMO or PPMO has been developed for in vivo application, however, either their toxicity or stability has become an issue. Previously, we reported nonpeptidic internal guanidinium transporter (IGT) mediated delivery of PMO for gene silencing and got encouraging results. In this paper, we report the synthesis of IGT using a Hg-free method for scale up and N-terminal modification of IGT with a suitable hydrophobic or lipophilic group to improve the cell permeability, endosomal escape, and mitochondrial localization and to reduce toxicity in the MTT assay. For the delivery of PMO, IGT-PMO conjugate was synthesized to target NANOG in cells, a transcription factor required for cancer stem cell proliferation and embryonic development and is involved in many cancers. Our data shows IGT-PMO-facilitated NANOG inhibition, and thereby the prevention of EpCAM-N-Cadherin-Vimentin axis mediated epithelial to mesenchymal transition (EMT) in MCF-7 cells. Moreover, unlike taxol, NANOG inhibition influences the expression of stemness factor c-Myc, Hh-Gli signaling proteins, other cancer related factors, and their respective phenotypes in cancer cells. To the best of our knowledge, this is the first report to illustrate that the IGT-PMO-mediated NANOG inhibition increases the therapeutic potential of taxol and induces G0-G1 arrest in cancer cells to prevent cancer progression. However, it warrants further investigation in other cancer cells and preclinical platforms.
中文翻译:

内部寡胍转运蛋白:无汞可扩展合成,细胞定位改善,内体逸出,线粒体定位和与反义吗啉结合对NANOG的抑制作用,从而诱导紫杉醇对MCF-7细胞的化学增敏作用。
对于反义二氨基氨基磷酸吗啉代寡核苷酸(PMO)的治疗应用,无毒的递送载体至关重要。尽管已经开发了体内结合的富胍基或精氨酸富集的细胞转运蛋白结合的Vivo-PMO或PPMO,但是它们的毒性或稳定性已成为一个问题。以前,我们报道了非肽内部胍转运蛋白(IGT)介导的PMO传递基因沉默,并获得令人鼓舞的结果。在本文中,我们报告了使用无汞方法进行IGT的合成以扩大规模和提高氮含量用合适的疏水或亲脂基团对IGT进行末端修饰,以改善细胞通透性,内体逸出和线粒体定位,并降低MTT分析的毒性。为了递送PMO,合成了IGT-PMO共轭物以靶向NANOG在细胞中,转录因子是癌症干细胞增殖和胚胎发育所必需的,并参与许多癌症。我们的数据显示,IGT-PMO促进了NANOG抑制,从而防止了MCF-7细胞中EpCAM-N-钙黏着蛋白-波形蛋白轴介导的上皮向间质转化(EMT)的发生。此外,与紫杉醇不同,NANOG抑制作用会影响干细胞因子c-Myc,Hh-Gli信号蛋白,其他癌症相关因子及其在癌细胞中的表型的表达。据我们所知,这是第一份说明IGT-PMO介导的NANOG抑制作用增加紫杉醇治疗潜力并诱导癌细胞中G0-G1阻滞以阻止癌症进展的报道。但是,它值得在其他癌细胞和临床前平台中进行进一步研究。
更新日期:2020-10-21
中文翻译:

内部寡胍转运蛋白:无汞可扩展合成,细胞定位改善,内体逸出,线粒体定位和与反义吗啉结合对NANOG的抑制作用,从而诱导紫杉醇对MCF-7细胞的化学增敏作用。
对于反义二氨基氨基磷酸吗啉代寡核苷酸(PMO)的治疗应用,无毒的递送载体至关重要。尽管已经开发了体内结合的富胍基或精氨酸富集的细胞转运蛋白结合的Vivo-PMO或PPMO,但是它们的毒性或稳定性已成为一个问题。以前,我们报道了非肽内部胍转运蛋白(IGT)介导的PMO传递基因沉默,并获得令人鼓舞的结果。在本文中,我们报告了使用无汞方法进行IGT的合成以扩大规模和提高氮含量用合适的疏水或亲脂基团对IGT进行末端修饰,以改善细胞通透性,内体逸出和线粒体定位,并降低MTT分析的毒性。为了递送PMO,合成了IGT-PMO共轭物以靶向NANOG在细胞中,转录因子是癌症干细胞增殖和胚胎发育所必需的,并参与许多癌症。我们的数据显示,IGT-PMO促进了NANOG抑制,从而防止了MCF-7细胞中EpCAM-N-钙黏着蛋白-波形蛋白轴介导的上皮向间质转化(EMT)的发生。此外,与紫杉醇不同,NANOG抑制作用会影响干细胞因子c-Myc,Hh-Gli信号蛋白,其他癌症相关因子及其在癌细胞中的表型的表达。据我们所知,这是第一份说明IGT-PMO介导的NANOG抑制作用增加紫杉醇治疗潜力并诱导癌细胞中G0-G1阻滞以阻止癌症进展的报道。但是,它值得在其他癌细胞和临床前平台中进行进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号