当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Chemical Probe for the Methyl Transferase PRMT5 with a Novel Binding Mode
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-09-28 , DOI: 10.1021/acsmedchemlett.0c00355 Vineet Pande 1 , Weimei Sun 2 , Lijs Beke 1 , Didier Berthelot 3 , Dirk Brehmer 1 , David Brown 4 , Jordi Corbera 5 , Steve Irving 4 , Lieven Meerpoel 1 , Thomas Nys 1 , Marc Parade 1 , Colin Robinson 4 , Cois Sommen 1 , Marcel Viellevoye 1 , Tongfei Wu 1 , Jan Willem Thuring 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-09-28 , DOI: 10.1021/acsmedchemlett.0c00355 Vineet Pande 1 , Weimei Sun 2 , Lijs Beke 1 , Didier Berthelot 3 , Dirk Brehmer 1 , David Brown 4 , Jordi Corbera 5 , Steve Irving 4 , Lieven Meerpoel 1 , Thomas Nys 1 , Marc Parade 1 , Colin Robinson 4 , Cois Sommen 1 , Marcel Viellevoye 1 , Tongfei Wu 1 , Jan Willem Thuring 1
Affiliation
|
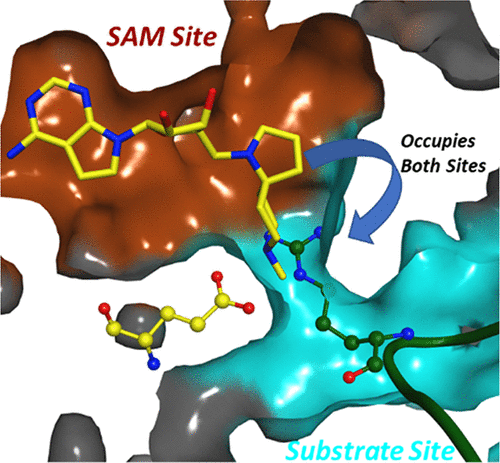
Protein arginine methyltransferase 5 (PRMT5) is an enzyme that can symmetrically dimethylate arginine residues in histones and nonhistone proteins by using S-adenosyl methionine (SAM) as the methyl donating cofactor. We have designed a library of SAM analogues and discovered potent, cell-active, and selective spiro diamines as inhibitors of the enzymatic function of PRMT5. Crystallographic studies confirmed a very interesting binding mode, involving protein flexibility, where both the cofactor pocket and part of substrate binding site are occupied by these inhibitors.
中文翻译:

具有新型结合模式的甲基转移酶 PRMT5 的化学探针
蛋白质精氨酸甲基转移酶 5 (PRMT5) 是一种酶,它可以使用S-腺苷甲硫氨酸 (SAM) 作为甲基供体辅助因子,对称地二甲基化组蛋白和非组蛋白中的精氨酸残基。我们设计了一个 SAM 类似物库,并发现了作为 PRMT5 酶功能抑制剂的强效、细胞活性和选择性螺二胺。晶体学研究证实了一种非常有趣的结合模式,涉及蛋白质灵活性,其中辅因子口袋和部分底物结合位点都被这些抑制剂占据。
更新日期:2020-11-12
中文翻译:

具有新型结合模式的甲基转移酶 PRMT5 的化学探针
蛋白质精氨酸甲基转移酶 5 (PRMT5) 是一种酶,它可以使用S-腺苷甲硫氨酸 (SAM) 作为甲基供体辅助因子,对称地二甲基化组蛋白和非组蛋白中的精氨酸残基。我们设计了一个 SAM 类似物库,并发现了作为 PRMT5 酶功能抑制剂的强效、细胞活性和选择性螺二胺。晶体学研究证实了一种非常有趣的结合模式,涉及蛋白质灵活性,其中辅因子口袋和部分底物结合位点都被这些抑制剂占据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号