当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The N-glycan profile in cortex and hippocampus is altered in Alzheimer disease
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-28 , DOI: 10.1111/jnc.15202 Stefan Gaunitz 1 , Lars O Tjernberg 1 , Sophia Schedin-Weiss 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-28 , DOI: 10.1111/jnc.15202 Stefan Gaunitz 1 , Lars O Tjernberg 1 , Sophia Schedin-Weiss 1
Affiliation
|
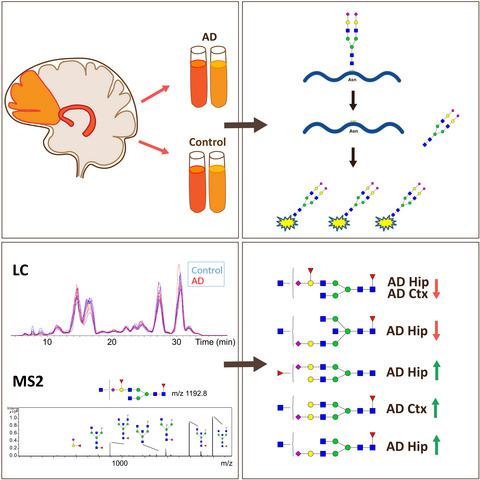
Protein glycosylation is crucial for the central nervous system and brain functions, including processes that are defective in Alzheimer disease (AD) such as neurogenesis, synaptic function, and memory formation. Still, the roles of glycans in the development of AD are relatively unexplored. Glycomics studies of cerebrospinal fluid (CSF) have previously shown altered glycosylation pattern in patients with different stages of cognitive impairment, including AD, compared to healthy controls. As a consequence, we hypothesized that the glycan profile is altered in the brain of patients with AD and analyzed the asparagine-linked (N-linked) glycan profile in hippocampus and cortex in AD and control brain. Glycans were enzymatically liberated from brain glycoproteins and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Eleven glycans showed significantly different levels in hippocampus compared to cortex in both control and AD brain. Two glycans in cortex and four in hippocampus showed different levels in AD compared to control brain. All glycans that differed between controls and AD brain had similar structures with one sialic acid, at least one fucose and a confirmed or potential bisecting N-acetylglucosamine (GlcNAc). The glycans that were altered in AD brain differed from those that were altered in AD CSF. One glycan found to be present in significantly lower levels in both hippocampus and cortex in AD compared to control contained a structurally and functionally interesting epitope that we assign as a terminal galactose decorated with fucose and sialic acid. Altogether, these studies suggest that protein glycosylation is an important component in the development of AD and warrants further studies.
中文翻译:

皮质和海马中的 N-聚糖谱在阿尔茨海默病中发生改变
蛋白质糖基化对中枢神经系统和大脑功能至关重要,包括阿尔茨海默病 (AD) 中存在缺陷的过程,如神经发生、突触功能和记忆形成。尽管如此,聚糖在 AD 发展中的作用还相对未被探索。脑脊液 (CSF) 的糖组学研究先前已经表明,与健康对照相比,包括 AD 在内的不同阶段的认知障碍患者的糖基化模式发生了改变。因此,我们假设 AD 患者大脑中的聚糖谱发生了改变,并分析了 AD 和对照大脑中海马和皮层中天冬酰胺相关(N 联)聚糖谱。聚糖是通过酶促从脑糖蛋白中释放出来的,并通过液相色谱-串联质谱 (LC-MS/MS) 进行分析。与对照和 AD 大脑中的皮层相比,海马中的 11 种聚糖显示出显着不同的水平。与对照大脑相比,皮层中的两种聚糖和海马体中的四种聚糖在 AD 中显示出不同的水平。对照和 AD 大脑之间存在差异的所有聚糖都具有相似的结构,具有一种唾液酸、至少一种岩藻糖和一种已确认或可能的二等分 N-乙酰氨基葡萄糖 (GlcNAc)。AD 大脑中改变的聚糖与 AD CSF 中改变的聚糖不同。与对照相比,在 AD 的海马和皮层中发现的一种聚糖含量显着降低,其中包含一个结构和功能上有趣的表位,我们将其指定为用岩藻糖和唾液酸装饰的末端半乳糖。共,
更新日期:2020-09-28
中文翻译:

皮质和海马中的 N-聚糖谱在阿尔茨海默病中发生改变
蛋白质糖基化对中枢神经系统和大脑功能至关重要,包括阿尔茨海默病 (AD) 中存在缺陷的过程,如神经发生、突触功能和记忆形成。尽管如此,聚糖在 AD 发展中的作用还相对未被探索。脑脊液 (CSF) 的糖组学研究先前已经表明,与健康对照相比,包括 AD 在内的不同阶段的认知障碍患者的糖基化模式发生了改变。因此,我们假设 AD 患者大脑中的聚糖谱发生了改变,并分析了 AD 和对照大脑中海马和皮层中天冬酰胺相关(N 联)聚糖谱。聚糖是通过酶促从脑糖蛋白中释放出来的,并通过液相色谱-串联质谱 (LC-MS/MS) 进行分析。与对照和 AD 大脑中的皮层相比,海马中的 11 种聚糖显示出显着不同的水平。与对照大脑相比,皮层中的两种聚糖和海马体中的四种聚糖在 AD 中显示出不同的水平。对照和 AD 大脑之间存在差异的所有聚糖都具有相似的结构,具有一种唾液酸、至少一种岩藻糖和一种已确认或可能的二等分 N-乙酰氨基葡萄糖 (GlcNAc)。AD 大脑中改变的聚糖与 AD CSF 中改变的聚糖不同。与对照相比,在 AD 的海马和皮层中发现的一种聚糖含量显着降低,其中包含一个结构和功能上有趣的表位,我们将其指定为用岩藻糖和唾液酸装饰的末端半乳糖。共,











































 京公网安备 11010802027423号
京公网安备 11010802027423号