当前位置:
X-MOL 学术
›
Luminescence
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of the nitrosyl ruthenium complex [RuII (NH.NHq‐R)(tpy)NO]3+ with human serum albumin: a spectroscopic and computational investigation
Luminescence ( IF 3.2 ) Pub Date : 2020-09-28 , DOI: 10.1002/bio.3955 Naiara Cristina Bessas 1, 2 , Letícia Alves da Silva 2 , Moacyr Comar Júnior 2 , Renata Galvão de Lima 1, 2
Luminescence ( IF 3.2 ) Pub Date : 2020-09-28 , DOI: 10.1002/bio.3955 Naiara Cristina Bessas 1, 2 , Letícia Alves da Silva 2 , Moacyr Comar Júnior 2 , Renata Galvão de Lima 1, 2
Affiliation
|
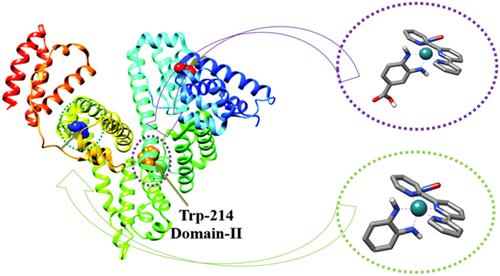
The interaction between two nitrosyl ruthenium complexes [Ru (NH.NHq–COOH)(tpy)NO](PF6)3 (RuBDQ) and [Ru (NH.NHq–H)(tpy)NO](PF6)3 (RuBD) and human serum albumin (HSA) was investigated using spectroscopic and computational methods. From fluorescence experiments, a dynamic quenching mechanism and binding constants at a single site demonstrated the higher stability of the RuBDQ–HSA system at 308 K compared with RuBD–HSA. Thermodynamic parameters indicated that binding of RuBDQ and RuBD to HSA was mainly driven by hydrophobic interaction and hydrogen bonding, respectively. Synchronous fluorescence and FT‐IR results suggested that interactions between both nitrosyl ruthenium complexes and HSA affected protein conformation. Competition experiments revealed that RuBDQ and RuBD bound to Sudlow sites I and II, respectively. Molecular docking results showed that RuBDQ interacted with Ser‐192 and Ala‐291 residues via hydrogen bonding and polar contact, respectively, whereas RuBD associated with Asn‐391 via a polar interaction. Noncovalent interaction results suggested that van der Waals interactions were the main binding forces for both systems, i.e. RuBDQ associated with Trp‐214 via van der Waals interaction and with Ty‐150 via dipole–dipole bonding, whereas RuBD associated with Tyr‐452 via van der Waals forces. The Asp‐391 residue interacted with the nitrosyl ligand via polar contact and the terpyridine ligand via van der Waals interaction.
中文翻译:

亚硝酰基钌络合物[RuII(NH.NHq-R)(tpy)NO] 3+与人血清白蛋白的相互作用:光谱学和计算研究
两个亚硝酰基钌配合物[Ru(NH.NHq–COOH)(tpy)NO](PF 6)3(RuBDQ)和[Ru(NH.NHq–H)(tpy)NO](PF 6)3之间的相互作用(RuBD)和人血清白蛋白(HSA)使用光谱和计算方法进行了研究。通过荧光实验,动态猝灭机制和单个位点的结合常数证明,RuBDQ-HSA系统在308 K时的稳定性高于RuBD-HSA。热力学参数表明RuBDQ和RuBD与HSA的结合分别主要由疏水相互作用和氢键驱动。同步荧光和FT-IR结果表明,亚硝酰钌配合物与HSA之间的相互作用会影响蛋白质构象。竞争实验表明,RuBDQ和RuBD分别与Sudlow位点I和II结合。分子对接结果表明,RuBDQ分别通过氢键和极性接触与Ser‐192和Ala‐291残基相互作用,而RuBD通过极性相互作用与Asn-391相关联。非共价相互作用的结果表明范德华相互作用是两个系统的主要结合力,即RuBDQ通过范德华相互作用与Trp-214缔合,通过偶极-偶极键合与Ty-150缔合,而RuBD与Van的Tyr-452缔合。德华力。Asp‐391残基通过极性接触与亚硝酰基配体相互作用,而叔吡啶配体通过范德华相互作用而相互作用。
更新日期:2020-09-28
中文翻译:

亚硝酰基钌络合物[RuII(NH.NHq-R)(tpy)NO] 3+与人血清白蛋白的相互作用:光谱学和计算研究
两个亚硝酰基钌配合物[Ru(NH.NHq–COOH)(tpy)NO](PF 6)3(RuBDQ)和[Ru(NH.NHq–H)(tpy)NO](PF 6)3之间的相互作用(RuBD)和人血清白蛋白(HSA)使用光谱和计算方法进行了研究。通过荧光实验,动态猝灭机制和单个位点的结合常数证明,RuBDQ-HSA系统在308 K时的稳定性高于RuBD-HSA。热力学参数表明RuBDQ和RuBD与HSA的结合分别主要由疏水相互作用和氢键驱动。同步荧光和FT-IR结果表明,亚硝酰钌配合物与HSA之间的相互作用会影响蛋白质构象。竞争实验表明,RuBDQ和RuBD分别与Sudlow位点I和II结合。分子对接结果表明,RuBDQ分别通过氢键和极性接触与Ser‐192和Ala‐291残基相互作用,而RuBD通过极性相互作用与Asn-391相关联。非共价相互作用的结果表明范德华相互作用是两个系统的主要结合力,即RuBDQ通过范德华相互作用与Trp-214缔合,通过偶极-偶极键合与Ty-150缔合,而RuBD与Van的Tyr-452缔合。德华力。Asp‐391残基通过极性接触与亚硝酰基配体相互作用,而叔吡啶配体通过范德华相互作用而相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号