NeuroToxicology ( IF 3.4 ) Pub Date : 2020-09-28 , DOI: 10.1016/j.neuro.2020.09.037 Alexandra Colón-Rodríguez 1 , Nicole M Colón-Carrión 2 , William D Atchison 1
|
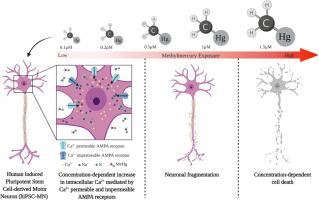
α motor neurons (MNs) are a target of the environmental neurotoxicant methylmercury (MeHg), accumulating MeHg and subsequently degenerating. In mouse spinal cord MN cultures, MeHg increased intracellular Ca2+ [Ca2+]i; the AMPA receptor (AMPAR) antagonist CNQX delayed the increase in [Ca2+]i, implicating the role of AMPARs in this response. Here we used human induced pluripotent stem cell-derived MNs (hiPSC-MNs), to characterize the role of MN AMPARs in MeHg neurotoxicity. Acute exposure to MeHg (0.1, 0.2, 0.5, 1 and 1.5 μM), fura-2 microfluorimetry, and a standard cytotoxicity assay, were used to examine MN regulation of [Ca2+]i, and cytotoxicity, respectively. Contribution of Ca2+-permeable and impermeable AMPARs was compared using either CNQX, or the Ca2+-permeable AMPAR antagonist N-acetyl spermine (NAS). MeHg-induced cytotoxicity was evaluated following a 24 h delay subsequent to 1 h exposure of hiPSC-MNs. MeHg caused a characteristic biphasic increase in [Ca2+]i, the onset of which was concentration-dependent; higher MeHg concentrations hastened onset of both phases. CNQX significantly delayed MeHg’s effect on onset time of both phases. In contrast, NAS significantly delayed only the 2nd phase increase in fura-2 fluorescence. Exposure to MeHg for 1 h followed by a 24 h recovery period caused a concentration-dependent incidence of cell death. These results demonstrate for the first time that hiPSC-derived MNs are highly sensitive to effects of MeHg on [Ca2+]i, and cytotoxicity, and that both Ca2+-permeable and impermeable AMPARs contribute the elevations in [Ca2+]i.
中文翻译:

AMPA受体对甲基汞介导的人诱导多能干细胞运动神经元细胞内Ca2+浓度改变的贡献
α 运动神经元 (MNs) 是环境神经毒物甲基汞 (MeHg) 的目标,可积累 MeHg 并随后退化。在小鼠脊髓 MN 培养物中,MeHg 增加细胞内 Ca 2+ [Ca 2+ ] i;AMPA 受体 (AMPAR) 拮抗剂 CNQX 延迟了 [Ca 2+ ] i的增加,暗示了 AMPAR 在该反应中的作用。在这里,我们使用人类诱导的多能干细胞衍生的 MNs (hiPSC-MNs) 来表征 MN AMPARs 在甲基汞神经毒性中的作用。急性暴露于 MeHg(0.1、0.2、0.5、1 和 1.5 μM)、fura-2 微量荧光法和标准细胞毒性测定,用于检查 [Ca 2+ ] i 的MN 调节,和细胞毒性,分别。使用 CNQX 或 Ca 2+可渗透 AMPAR 拮抗剂 N-乙酰精胺 (NAS)比较Ca 2+可渗透和不可渗透 AMPAR 的贡献。在 hiPSC-MNs 暴露 1 小时后延迟 24 小时评估甲基汞诱导的细胞毒性。MeHg 导致 [Ca 2+ ] i的特征性双相增加,其发作是浓度依赖性的;较高的甲基汞浓度加速了两个阶段的开始。CNQX 显着延迟了 MeHg 对两个阶段开始时间的影响。相比之下,NAS 仅显着延迟了 fura-2 荧光的第二阶段增加。暴露于甲基汞 1 小时,然后是 24 小时的恢复期,导致细胞死亡的浓度依赖性发生率。这些结果首次表明,hiPSC 衍生的 MNs 对 MeHg 对 [Ca 2+ ] i和细胞毒性的影响高度敏感,并且 Ca 2+可渗透和不可渗透的 AMPAR均有助于 [Ca 2+ ]的升高我。











































 京公网安备 11010802027423号
京公网安备 11010802027423号