当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of Interleukin-1β and Reduction in Atherothrombotic Cardiovascular Events in the CANTOS Trial
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.jacc.2020.08.011 Brendan M. Everett , Jean G. MacFadyen , Tom Thuren , Peter Libby , Robert J. Glynn , Paul M Ridker
Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.jacc.2020.08.011 Brendan M. Everett , Jean G. MacFadyen , Tom Thuren , Peter Libby , Robert J. Glynn , Paul M Ridker
|
BACKGROUND
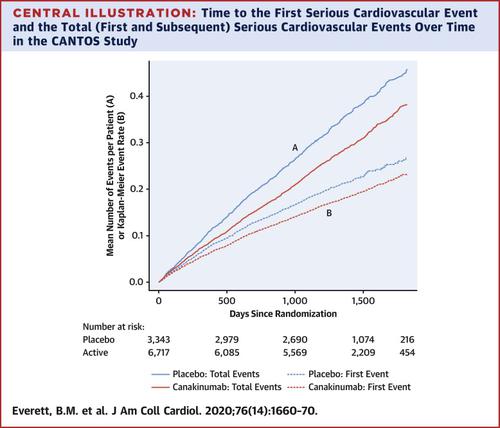
Inflammation reduction with the interleukin (IL)-1β inhibitor canakinumab significantly reduces the first major adverse cardiovascular event in patients with prior myocardial infarction (MI) and residual inflammatory risk (high-sensitivity C-reactive protein ≥ 2 mg/l). However, the effect of canakinumab on the total number of cardiovascular events, including recurrent events collected after a first event, is unknown. OBJECTIVES
This study sought to determine whether randomly allocated canakinumab would reduce the total burden of serious cardiovascular events. METHODS
We randomized 10,061 patients to placebo or canakinumab 50 mg, 150 mg, or 300 mg once every 3 months and compared the rates of the composite of all serious cardiovascular events (MI, stroke, coronary revascularization, and cardiovascular death) in active versus placebo groups. We used negative binomial regression to account for correlations among repeated events in the same person and to estimate rate ratios and 95% confidence intervals. RESULTS
During a median of 3.7 years of follow-up, 3,417 total serious cardiovascular events occurred in 2,003 individuals among the 10,061 unique patients randomized. Canakinumab reduced the rates of total serious cardiovascular events, with rates per 100 person-years in the placebo, 50 mg, 150 mg, and 300 mg canakinumab groups of 10.4, 8.4, 8.3, and 8.2, respectively. The corresponding rate ratios (95% confidence intervals) compared with placebo were 0.80 (0.69 to 0.93) for 50 mg, 0.79 (0.68 to 0.92) for 150 mg, and 0.78 (0.67 to 0.91) for 300 mg. CONCLUSIONS
Anti-inflammatory therapy with canakinumab significantly reduced the total number of cardiovascular events in patients with prior MI and evidence of residual inflammatory risk. (Cardiovascular Risk Reduction Study [Reduction in Recurrent Major CV Disease Events] (CANTOS); NCT01327846.
中文翻译:

在 CANTOS 试验中抑制白细胞介素 1β 和减少动脉粥样硬化血栓形成的心血管事件
背景 使用白介素 (IL)-1β 抑制剂卡那奴单抗减少炎症可显着降低既往心肌梗死 (MI) 和残留炎症风险(高敏 C 反应蛋白 ≥ 2 mg/l)患者的首个主要不良心血管事件。然而,canakinumab 对心血管事件总数(包括首次事件后收集的复发事件)的影响尚不清楚。目的 本研究旨在确定随机分配的卡那奴单抗是否会降低严重心血管事件的总负担。方法 我们将 10,061 名患者随机分配接受安慰剂或卡那奴单抗 50 mg、150 mg 或 300 mg,每 3 个月一次,并比较所有严重心血管事件(MI、中风、冠状动脉血运重建、和心血管死亡)在活性组与安慰剂组中。我们使用负二项式回归来解释同一个人重复事件之间的相关性,并估计比率和 95% 置信区间。结果 在中位 3.7 年的随访期间,随机分配的 10,061 名独特患者中的 2,003 人共发生了 3,417 次严重心血管事件。Canakinumab 降低了总严重心血管事件的发生率,安慰剂组、50 mg、150 mg 和 300 mg canakinumab 组的每 100 人年发生率分别为 10.4、8.4、8.3 和 8.2。与安慰剂相比,50 毫克的相应比率(95% 置信区间)为 0.80(0.69 至 0.93),150 毫克为 0.79(0.68 至 0.92),300 毫克为 0.78(0.67 至 0.91)。结论 卡那奴单抗抗炎治疗显着减少了既往 MI 和有残余炎症风险证据的患者的心血管事件总数。(心血管风险降低研究 [减少复发性主要心血管疾病事件] (CANTOS);NCT01327846。
更新日期:2020-10-01
中文翻译:

在 CANTOS 试验中抑制白细胞介素 1β 和减少动脉粥样硬化血栓形成的心血管事件
背景 使用白介素 (IL)-1β 抑制剂卡那奴单抗减少炎症可显着降低既往心肌梗死 (MI) 和残留炎症风险(高敏 C 反应蛋白 ≥ 2 mg/l)患者的首个主要不良心血管事件。然而,canakinumab 对心血管事件总数(包括首次事件后收集的复发事件)的影响尚不清楚。目的 本研究旨在确定随机分配的卡那奴单抗是否会降低严重心血管事件的总负担。方法 我们将 10,061 名患者随机分配接受安慰剂或卡那奴单抗 50 mg、150 mg 或 300 mg,每 3 个月一次,并比较所有严重心血管事件(MI、中风、冠状动脉血运重建、和心血管死亡)在活性组与安慰剂组中。我们使用负二项式回归来解释同一个人重复事件之间的相关性,并估计比率和 95% 置信区间。结果 在中位 3.7 年的随访期间,随机分配的 10,061 名独特患者中的 2,003 人共发生了 3,417 次严重心血管事件。Canakinumab 降低了总严重心血管事件的发生率,安慰剂组、50 mg、150 mg 和 300 mg canakinumab 组的每 100 人年发生率分别为 10.4、8.4、8.3 和 8.2。与安慰剂相比,50 毫克的相应比率(95% 置信区间)为 0.80(0.69 至 0.93),150 毫克为 0.79(0.68 至 0.92),300 毫克为 0.78(0.67 至 0.91)。结论 卡那奴单抗抗炎治疗显着减少了既往 MI 和有残余炎症风险证据的患者的心血管事件总数。(心血管风险降低研究 [减少复发性主要心血管疾病事件] (CANTOS);NCT01327846。










































 京公网安备 11010802027423号
京公网安备 11010802027423号