Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-09-28 , DOI: 10.1016/j.bbagen.2020.129753 I. Scurci , K.B. Akondi , I. Pinheiro , M. Paolini-Bertrand , A. Borgeat , F. Cerini , O. Hartley
|
Background
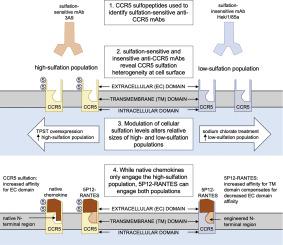
Chemokine receptor tyrosine sulfation plays a key role in the binding of chemokines. It has been suggested that receptor sulfation is heterogeneous, but no experimental evidence has been provided so far. The potent anti-HIV chemokine analog 5P12-RANTES has been proposed to owe its inhibitory activity to a capacity to bind a larger pool of cell surface CCR5 receptors than native chemokines such as CCL5, but the molecular details underlying this phenomenon have not been elucidated.
Methods
We investigated the CCR5 sulfation heterogeneity and the sensitivity of CCR5 ligands to receptor sulfation by performing ELISA assays on synthetic N-terminal sulfopeptides and by performing binding assays on CCR5-expressing cells under conditions that modulate CCR5 sulfation levels.
Results
Two commonly used anti-CCR5 monoclonal antibodies with epitopes in the sulfated N-terminal domain of CCR5 show contrasting binding profiles on CCR5 sulfopeptides, incomplete competition with each other for cell surface CCR5, and opposing sensitivities to cellular treatments that affect CCR5 sulfation levels. 5P12-RANTES is less sensitive than native CCL5 to conditions that affect cellular CCR5 sulfation.
Conclusions
CCR5 sulfation is heterogeneous and this affects the binding properties of both native chemokines and antibodies. Enhanced capacity to bind to CCR5 is a component of the inhibitory mechanism of 5P12-RANTES.
General significance
We provide the first experimental evidence for sulfation heterogeneity of chemokine receptors and its impact on ligand binding, a phenomenon that is important both for the understanding of chemokine cell biology and for the development of drugs that target chemokine receptors.
中文翻译:

CCR5酪氨酸硫酸化异质性产生具有不同配体结合特性的细胞表面受体亚群
背景
趋化因子受体酪氨酸硫酸化在趋化因子的结合中起关键作用。已经提出受体硫酸化是异质的,但是到目前为止还没有提供实验证据。已提出有效的抗HIV趋化因子类似物5P12-RANTES具有抑制活性,因为它具有比天然趋化因子(如CCL5)结合更大的细胞表面CCR5受体库的能力,但尚未阐明这种现象的分子细节。
方法
我们研究了CCR5硫酸盐异质性和CCR5配体对受体硫酸盐敏感性的敏感性,方法是对合成的N末端硫肽进行ELISA分析,并在调节CCR5硫酸盐水平的条件下对表达CCR5的细胞进行结合分析。
结果
两种常用的抗CCR5单克隆抗体的CCR5的硫酸化N末端结构域具有表位,显示在CCR5硫肽上的结合曲线不同,彼此对细胞表面CCR5的竞争不完全以及对影响CCR5硫酸化水平的细胞治疗的敏感性相反。5P12-RANTES对影响细胞CCR5硫酸化的条件的敏感性低于天然CCL5。
结论
CCR5硫酸化作用是异质的,这会影响天然趋化因子和抗体的结合特性。结合CCR5的能力增强是5P12-RANTES抑制机制的组成部分。
一般意义
我们提供了趋化因子受体的硫酸盐异质性及其对配体结合的影响的第一个实验证据,这种现象对于理解趋化因子细胞生物学和开发靶向趋化因子受体的药物都具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号