当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Tandem Carbonylative Aza‐Wacker‐Type Cyclization of Nucleophile Tethered Alkene to Access Fused N‐Heterocycles
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-09-27 , DOI: 10.1002/cjoc.202000491 Lijun Shi 1, 2 , Mingshan Wen 1 , Fuwei Li 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-09-27 , DOI: 10.1002/cjoc.202000491 Lijun Shi 1, 2 , Mingshan Wen 1 , Fuwei Li 1
Affiliation
|
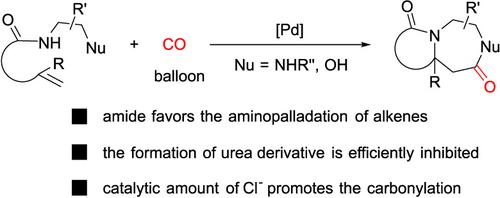
Although tandem reactions offer rapid access to structurally complex molecules in one‐pot reaction, the selectivity issue needs to be addressed particularly when incompatible step reactions are involved. Herein, we report the selective synthesis of fused N‐heterocycles from nucleophile‐tethered alkenylamide and carbon monoxide via palladium (Pd)‐catalyzed tandem carbonylative aza‐Wacker‐type cyclization. The electron‐deficient nature of amide N—H and the intramolecular coordination of Pd with alkene accelerate the aminopalladation and effectively prevent the side oxidative carbonylation of diamine moiety to form urea. It is also found that the reported acyl Pd chloride intermediate may not be involved in this tandem cyclization. This work not only provides an efficient synthetic route to fused 1,4‐diazepanones and 1,4‐diazepanes but also inspires further development of tandem reactions for the diverse synthesis of heterocycles.
中文翻译:

亲核分子链烷烃的钯催化串联羰基化氮杂-瓦克型环化反应,以获取熔融的N-杂环
尽管串联反应可在一锅反应中快速接近结构复杂的分子,但选择性问题仍需解决,特别是涉及不相容的阶跃反应时。在此,我们报道了通过亲核连接的链烯基酰胺和一氧化碳通过以下方法选择性合成稠合的N-杂环钯(Pd)催化的串联羰基化氮杂-瓦克型环化反应。酰胺NH的电子不足性质以及Pd与烯烃的分子内配位会加速氨基palpalation,并有效防止二胺部分的侧氧化羰基化形成尿素。还发现报道的酰基Pd氯化物中间体可能不参与该串联环化。这项工作不仅为融合1,4-二氮杂潘酮和1,4-二氮杂潘酮提供了有效的合成途径,而且还激发了串联反应的进一步发展,以实现杂环的多样化合成。
更新日期:2020-09-27

中文翻译:

亲核分子链烷烃的钯催化串联羰基化氮杂-瓦克型环化反应,以获取熔融的N-杂环
尽管串联反应可在一锅反应中快速接近结构复杂的分子,但选择性问题仍需解决,特别是涉及不相容的阶跃反应时。在此,我们报道了通过亲核连接的链烯基酰胺和一氧化碳通过以下方法选择性合成稠合的N-杂环钯(Pd)催化的串联羰基化氮杂-瓦克型环化反应。酰胺NH的电子不足性质以及Pd与烯烃的分子内配位会加速氨基palpalation,并有效防止二胺部分的侧氧化羰基化形成尿素。还发现报道的酰基Pd氯化物中间体可能不参与该串联环化。这项工作不仅为融合1,4-二氮杂潘酮和1,4-二氮杂潘酮提供了有效的合成途径,而且还激发了串联反应的进一步发展,以实现杂环的多样化合成。












































 京公网安备 11010802027423号
京公网安备 11010802027423号