当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequential Organocatalytic Synthesis of [1,2,3]Triazolo[1,5‐a]quinolines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-25 , DOI: 10.1002/adsc.202000887 Gabriel Costa 1 , Mariana Bach 1 , Maiara de Moraes 2 , Thiago Barcellos da Silva 2 , Eder Lenardao 3 , Márcio Silva 3 , Diego Alves 4
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-25 , DOI: 10.1002/adsc.202000887 Gabriel Costa 1 , Mariana Bach 1 , Maiara de Moraes 2 , Thiago Barcellos da Silva 2 , Eder Lenardao 3 , Márcio Silva 3 , Diego Alves 4
Affiliation
|
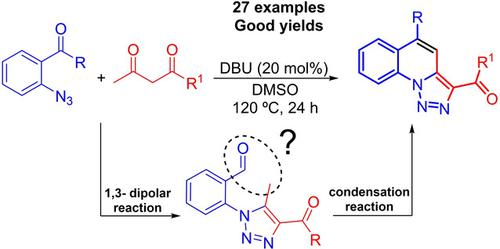
In this work, a one‐pot sequential organocatalytic method for the synthesis of fused [1,2,3]triazolo[1,5‐a]quinolines through successive cyclization and condensation is presented. In this synthetic strategy, the intermolecular [3+2]‐cycloaddition occurs between 1,3‐dicarbonyl compounds and o‐carbonyl‐substituted phenylazide compounds, for the formation of the 1,2,3‐triazole intermediates. Subsequently, an intramolecular condensation reaction generates the fused quinoline ring by the new C−C bond formation, giving the products in yields ranging from moderate to excellent. All the reactions were performed using 20 mol% of 1,8‐diazabicyclo[5.4.0]undec‐7‐ene (DBU) as catalyst in the presence of DMSO as solvent at 120 °C for 24 h and tolerate a range of 1,3‐dicarbonyl compounds, such as β‐keto esters and 1,3‐diketones, and o‐formyl, o‐acetyl or o‐benzoyl substituted phenylazide compounds.
中文翻译:

[1,2,3]三唑并[1,5-a]喹啉的顺序有机催化合成
在这项工作中,提出了一种通过连续环化和缩合反应合成稠合[1,2,3]三唑并[1,5- a ]喹啉的单锅顺序有机催化方法。在这种合成策略中,分子间的[3 + 2]-环加成反应发生在1,3-二羰基化合物与邻羰基取代的苯叠氮化合物之间,从而形成1,2,3-三唑中间体。随后,分子内的缩合反应通过新的C-C键的形成生成稠合的喹啉环,从而使产物的收率从中等到极好。在DMSO作为溶剂存在下,使用20 mol%的1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)作为催化剂,在120°C下进行24 h,反应范围为1。 ,3-二羰基化合物,例如β-酮酸酯和1,3-二酮,以及邻甲酰基,邻乙酰基或邻苯甲酰基取代的苯叠氮化合物。
更新日期:2020-11-19
中文翻译:

[1,2,3]三唑并[1,5-a]喹啉的顺序有机催化合成
在这项工作中,提出了一种通过连续环化和缩合反应合成稠合[1,2,3]三唑并[1,5- a ]喹啉的单锅顺序有机催化方法。在这种合成策略中,分子间的[3 + 2]-环加成反应发生在1,3-二羰基化合物与邻羰基取代的苯叠氮化合物之间,从而形成1,2,3-三唑中间体。随后,分子内的缩合反应通过新的C-C键的形成生成稠合的喹啉环,从而使产物的收率从中等到极好。在DMSO作为溶剂存在下,使用20 mol%的1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU)作为催化剂,在120°C下进行24 h,反应范围为1。 ,3-二羰基化合物,例如β-酮酸酯和1,3-二酮,以及邻甲酰基,邻乙酰基或邻苯甲酰基取代的苯叠氮化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号