当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic and Thermodynamic Behaviors of Pseudorotaxane Formation with C3v Macrocyclic BODIPY Trimers and the Remarkable Substituent Effect on Ring-Face Selectivity.
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-25 , DOI: 10.1021/acs.orglett.0c02840 Ryota Matsuoka 1 , Sou Himori 1 , Gento Yamaguchi 1 , Tatsuya Nabeshima 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-09-25 , DOI: 10.1021/acs.orglett.0c02840 Ryota Matsuoka 1 , Sou Himori 1 , Gento Yamaguchi 1 , Tatsuya Nabeshima 1
Affiliation
|
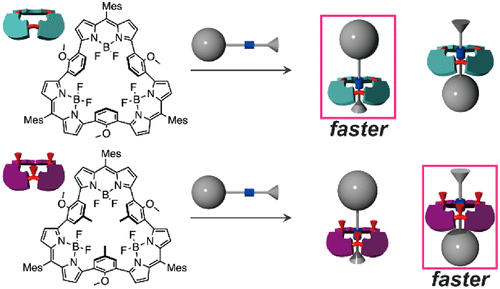
The thermodynamic and kinetic behaviors of the pseudorotaxane formation between the C3v macrocyclic BODIPY trimers and unsymmetrical secondary ammonium guests are investigated. We find a remarkable substituent effect of the BODIPY trimer on the ring-face selectivity during the threading. The difference in the small substituents (H or CH3) in the macrocyclic host molecules significantly modulated the thermodynamic and kinetic selectivity of the threading direction of the unsymmetrical ammonium ions.
中文翻译:

C3v大环BODIPY三聚体形成伪轮烷的动力学和热力学行为以及对环表面选择性的显着取代基作用。
研究了C 3v大环BODIPY三聚体和不对称仲铵客体之间假轮烷形成的热力学和动力学行为。我们发现穿线过程中BODIPY三聚体对环表面选择性的显着取代作用。大环主体分子中小的取代基(H或CH 3)的差异显着调节了不对称铵离子穿入方向的热力学和动力学选择性。
更新日期:2020-11-21
中文翻译:

C3v大环BODIPY三聚体形成伪轮烷的动力学和热力学行为以及对环表面选择性的显着取代基作用。
研究了C 3v大环BODIPY三聚体和不对称仲铵客体之间假轮烷形成的热力学和动力学行为。我们发现穿线过程中BODIPY三聚体对环表面选择性的显着取代作用。大环主体分子中小的取代基(H或CH 3)的差异显着调节了不对称铵离子穿入方向的热力学和动力学选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号