当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deficiency of the serine peptidase Kallikrein 6 does not affect the levels and the pathological accumulation of a-synuclein in mouse brain
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-24 , DOI: 10.1111/jnc.15199 Vasia Samantha Sykioti 1 , Mantia Karampetsou 1 , Ioanna Chalatsa 1, 2 , Alexia Polissidis 1 , Iacovos P Michael 3 , Marina Pagaki-Skaliora 1 , Andras Nagy 3, 4 , Evangelia Emmanouilidou 1, 2 , Georgia Sotiropoulou 5 , Kostas Vekrelli S 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-09-24 , DOI: 10.1111/jnc.15199 Vasia Samantha Sykioti 1 , Mantia Karampetsou 1 , Ioanna Chalatsa 1, 2 , Alexia Polissidis 1 , Iacovos P Michael 3 , Marina Pagaki-Skaliora 1 , Andras Nagy 3, 4 , Evangelia Emmanouilidou 1, 2 , Georgia Sotiropoulou 5 , Kostas Vekrelli S 1
Affiliation
|
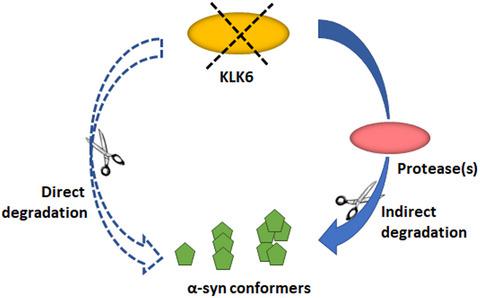
Several lines of evidence indicate that the propagation of misfolded α-synuclein (α-syn) plays a central role in the progression and manifestation of Parkinson's disease. Pathogenic α-syn species can be present in the extracellular space. Thus, the identification and modulation of the key enzymes implicated in extracellular α-syn turnover becomes vital. Kallikrein peptidase 6 has been identified as one of the major α-syn degrading enzymes and has been implicated in the clearance of extracellular α-syn. However, the physiological role of this enzyme in regulating α-syn, in vivo, still remains elusive. Here, by utilizing Klk6 knock-out (Klk6−/−) mice as our experimental model, we provide insight into the physiologic relevance of endogenous KLK6 expression on α-syn processing. Behavioral phenotyping showed that Klk6−/− mice display no gross behavioral abnormalities. Further in vivo characterization of this mouse model, in the context of α-syn accumulation, showed that KLK6 deletion had no impact on the protein levels of intracellular or extracellular α-syn. Upon in vivo administration of α-syn pre-formed fibrils (PFF), α-syn pathologic accumulations were evident both in the brains of Klk6−/−mice and wt mice without significant differences. Intrastriatal delivery of active KLK6, did not affect secreted α-syn levels observed in the A53T α-syn over-expressing mice. These findings suggest that in the in vivo setting of PFF pathology induction, KLK6 alone is not able to modulate pathology transmission. Our study raises implications for the use of recombinant α-syn fibrils in α-syn turnover studies.
中文翻译:

丝氨酸肽酶激肽释放酶6的缺乏不影响小鼠脑中α-突触核蛋白的水平和病理积累
多项证据表明,错误折叠的 α-突触核蛋白 (α-syn) 的传播在帕金森病的进展和表现中起着核心作用。致病性 α-syn 物种可以存在于细胞外空间。因此,鉴定和调节与细胞外 α-syn 转换有关的关键酶变得至关重要。激肽释放酶肽酶 6 已被鉴定为主要的 α-syn 降解酶之一,并与细胞外 α-syn 的清除有关。然而,这种酶在体内调节 α-syn 的生理作用仍然难以捉摸。在这里,通过利用 Klk6 敲除(Klk6 -/-) 小鼠作为我们的实验模型,我们深入了解内源性 KLK6 表达对 α-syn 加工的生理相关性。行为表型显示Klk6 -/-小鼠没有表现出明显的行为异常。在 α-syn 积累的背景下,对该小鼠模型的进一步体内表征表明,KLK6 缺失对细胞内或细胞外 α-syn 的蛋白质水平没有影响。在体内施用 α-syn 预先形成的原纤维 (PFF) 后,在Klk6 -/-的大脑中都明显存在α-syn 病理性积累小鼠和wt小鼠无显着差异。活性 KLK6 的纹状体内递送不影响在 A53T α-syn 过表达小鼠中观察到的分泌 α-syn 水平。这些发现表明,在 PFF 病理诱导的体内环境中,单独的 KLK6 不能调节病理传递。我们的研究对在 α-syn 转换研究中使用重组 α-syn 原纤维提出了启示。
更新日期:2020-09-24
中文翻译:

丝氨酸肽酶激肽释放酶6的缺乏不影响小鼠脑中α-突触核蛋白的水平和病理积累
多项证据表明,错误折叠的 α-突触核蛋白 (α-syn) 的传播在帕金森病的进展和表现中起着核心作用。致病性 α-syn 物种可以存在于细胞外空间。因此,鉴定和调节与细胞外 α-syn 转换有关的关键酶变得至关重要。激肽释放酶肽酶 6 已被鉴定为主要的 α-syn 降解酶之一,并与细胞外 α-syn 的清除有关。然而,这种酶在体内调节 α-syn 的生理作用仍然难以捉摸。在这里,通过利用 Klk6 敲除(Klk6 -/-) 小鼠作为我们的实验模型,我们深入了解内源性 KLK6 表达对 α-syn 加工的生理相关性。行为表型显示Klk6 -/-小鼠没有表现出明显的行为异常。在 α-syn 积累的背景下,对该小鼠模型的进一步体内表征表明,KLK6 缺失对细胞内或细胞外 α-syn 的蛋白质水平没有影响。在体内施用 α-syn 预先形成的原纤维 (PFF) 后,在Klk6 -/-的大脑中都明显存在α-syn 病理性积累小鼠和wt小鼠无显着差异。活性 KLK6 的纹状体内递送不影响在 A53T α-syn 过表达小鼠中观察到的分泌 α-syn 水平。这些发现表明,在 PFF 病理诱导的体内环境中,单独的 KLK6 不能调节病理传递。我们的研究对在 α-syn 转换研究中使用重组 α-syn 原纤维提出了启示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号