当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Catalyzed Direct Trifluoroethylation of Aryl Iodides with 1,1,1‐Trifluoro‐2‐Iodoethane via Reductive Coupling
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-24 , DOI: 10.1002/adsc.202000985 Han Li 1 , Jie Sheng 1 , Guang-Xu Liao 1 , Bing-Bing Wu 1 , Hui-Qi Ni 1 , Yan Li 1 , Xi-Sheng Wang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-24 , DOI: 10.1002/adsc.202000985 Han Li 1 , Jie Sheng 1 , Guang-Xu Liao 1 , Bing-Bing Wu 1 , Hui-Qi Ni 1 , Yan Li 1 , Xi-Sheng Wang 1
Affiliation
|
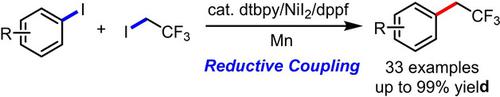
A nickel‐catalyzed direct trifluoroethylation of aryl iodides with an industrial raw material CF3CH2I has been developed, demonstrating high efficiency, excellent functional‐group compatibility, especially with large sterically hindered groups. The key to success is the combination of nickel with readily available nitrogen and phosphine ligands. The powerful potential of this strategy is further demonstrated by the late‐stage modification of several derived bioactive molecules.
中文翻译:

镍通过1,1,1,1-三氟-2-碘乙烷与芳基碘化物的镍直接三氟乙基化反应
已经开发出了镍与工业原料CF 3 CH 2 I催化的碘代芳基碘的直接三氟乙基化反应,显示出高效率,出色的官能团相容性,特别是对于大的位阻基团。成功的关键是镍与现成的氮和膦配体的结合。几种衍生生物活性分子的后期修饰进一步证明了该策略的强大潜力。
更新日期:2020-09-24
中文翻译:

镍通过1,1,1,1-三氟-2-碘乙烷与芳基碘化物的镍直接三氟乙基化反应
已经开发出了镍与工业原料CF 3 CH 2 I催化的碘代芳基碘的直接三氟乙基化反应,显示出高效率,出色的官能团相容性,特别是对于大的位阻基团。成功的关键是镍与现成的氮和膦配体的结合。几种衍生生物活性分子的后期修饰进一步证明了该策略的强大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号