当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dienolate‐Mediated, Regioselective C2‐Polarity Reversal of Chromone‐Based Reactants and Their Application in Nucleophilic Strategies
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-24 , DOI: 10.1002/adsc.202000942 Zhen Yao 1 , Xiong-Wei Liu 2 , Zheng Li 1 , Sheng-Wen Xu 1 , Lijin Xu 3 , Xiong-Li Liu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-24 , DOI: 10.1002/adsc.202000942 Zhen Yao 1 , Xiong-Wei Liu 2 , Zheng Li 1 , Sheng-Wen Xu 1 , Lijin Xu 3 , Xiong-Li Liu 1
Affiliation
|
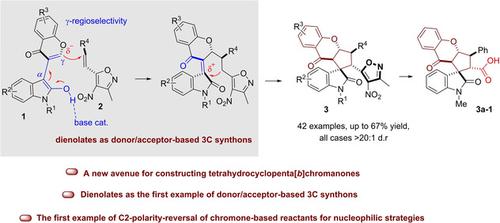
Inspired by the chemistry and biology of chromone, a method for the construction of spirooxindole‐based tetrahydrocyclopenta[b]chromanones is reported with good regio‐ and diastereoselectivity (all cases >20:1 dr) and moderate yields (up to 67%). This method leads to the construction of four contiguous stereogenic tertiary centers and one spiro quaternary center via a one‐step γ‐regioselective cascade [3+2] cycloaddition of an oxindole‐chromone synthon with 3‐methyl‐4‐nitro‐5‐alkenyl‐isoxazole.
中文翻译:

苯甲酸酯基反应物的二烯酸酯介导的区域选择性C2极性逆转及其在亲核策略中的应用
受色酮化学和生物学的启发,据报道,一种基于螺氧杂吲哚的四氢环戊四烯[ b ]色酮的构建方法具有良好的区域选择性和非对映选择性(所有情况> 20:1 dr)和中等产率(最高67%)。该方法通过一个羟吲哚-色酮合成子与3-甲基-4-4-硝基-5-烯基的一步式γ-区域选择性级联[3 + 2]环加成反应,从而构建了四个连续的立体成因三级中心和一个螺旋四级中心。异唑
更新日期:2020-09-24
中文翻译:

苯甲酸酯基反应物的二烯酸酯介导的区域选择性C2极性逆转及其在亲核策略中的应用
受色酮化学和生物学的启发,据报道,一种基于螺氧杂吲哚的四氢环戊四烯[ b ]色酮的构建方法具有良好的区域选择性和非对映选择性(所有情况> 20:1 dr)和中等产率(最高67%)。该方法通过一个羟吲哚-色酮合成子与3-甲基-4-4-硝基-5-烯基的一步式γ-区域选择性级联[3 + 2]环加成反应,从而构建了四个连续的立体成因三级中心和一个螺旋四级中心。异唑











































 京公网安备 11010802027423号
京公网安备 11010802027423号