当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of Catalyst Structure for Asymmetric Propargylation of Aldehydes with Allenyltrichlorosilane
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-24 , DOI: 10.1002/adsc.202000936 Vladimir Vaganov 1 , Yasuaki Fukazawa 2 , Nikolay Kondratyev 2 , Sergei Shipilovskikh 1 , Steven Wheeler 3 , Aleksandr Rubtsov 1 , Andrei V. Malkov 4
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-24 , DOI: 10.1002/adsc.202000936 Vladimir Vaganov 1 , Yasuaki Fukazawa 2 , Nikolay Kondratyev 2 , Sergei Shipilovskikh 1 , Steven Wheeler 3 , Aleksandr Rubtsov 1 , Andrei V. Malkov 4
Affiliation
|
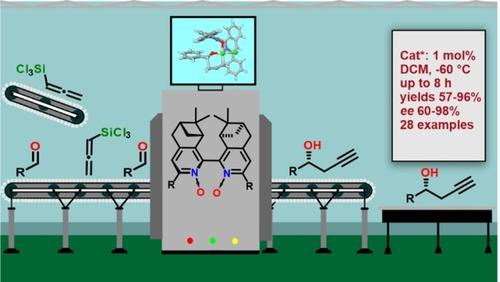
The design of catalysts for asymmetric propargylations remains a challenging task, with only a handful of methods providing access to enantioenriched homopropargylic alcohols. In this work, guided by previously reported computational predictions, a set of atropisomeric bipyridine N,N’‐dioxides was tested as Lewis base catalysts for the asymmetric propargylation of aldehydes with trichloroallenylsilane. The catalysts are easily prepared in four simple steps starting from readily available methyl ketones. Aryl‐substituted derivatives proved to be highly active and showed a high level of enantiocontrol even at 1 mol% loading. The reaction scope includes a wide range of aromatic, heteroaromatic, and unsaturated aldehydes. New computations confirm that the key stereodetermining transition state structures for the synthesized catalysts are similar to those previously reported for the model structure.
中文翻译:

烯丙基三氯硅烷不对称丙醛丙炔化催化剂结构的优化
用于不对称炔丙基化的催化剂的设计仍然是一项艰巨的任务,只有少数几种方法可提供对映体富集的均炔丙醇。在这项工作中,在先前报道的计算预测的指导下,一组阻转异构联吡啶N,N'二氧化物被用作路易斯碱催化剂,用于醛与三氯烯丙基硅烷的不对称炔丙基化反应。从容易获得的甲基酮开始,可通过四个简单的步骤轻松制备催化剂。芳基取代的衍生物被证明具有很高的活性,即使在1 mol%的负载量下也表现出高水平的对映体控制。反应范围包括多种芳族,杂芳族和不饱和醛。新的计算证实了合成催化剂的关键立体确定过渡态结构与先前报道的模型结构相似。
更新日期:2020-09-24
中文翻译:

烯丙基三氯硅烷不对称丙醛丙炔化催化剂结构的优化
用于不对称炔丙基化的催化剂的设计仍然是一项艰巨的任务,只有少数几种方法可提供对映体富集的均炔丙醇。在这项工作中,在先前报道的计算预测的指导下,一组阻转异构联吡啶N,N'二氧化物被用作路易斯碱催化剂,用于醛与三氯烯丙基硅烷的不对称炔丙基化反应。从容易获得的甲基酮开始,可通过四个简单的步骤轻松制备催化剂。芳基取代的衍生物被证明具有很高的活性,即使在1 mol%的负载量下也表现出高水平的对映体控制。反应范围包括多种芳族,杂芳族和不饱和醛。新的计算证实了合成催化剂的关键立体确定过渡态结构与先前报道的模型结构相似。










































 京公网安备 11010802027423号
京公网安备 11010802027423号