当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal preparation and characterization of nanodispersed copper-containing powders produced by non-equilibrium electrochemical oxidation of metals
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.solidstatesciences.2020.106434 Valeriy V. Korobochkin , Johannes H. Potgieter , Natalia V. Usoltseva , Alesya S. Dolinina , Vladimir V. An
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.solidstatesciences.2020.106434 Valeriy V. Korobochkin , Johannes H. Potgieter , Natalia V. Usoltseva , Alesya S. Dolinina , Vladimir V. An
|
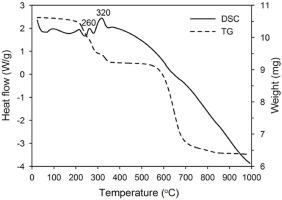
Abstract Physicochemical properties of copper, aluminum and cadmium oxides determine their application in different ways. Electrochemical metal oxidation using alternating current (AC) influences the band gap of prepared copper-containing oxide materials and result in a blue shift. The use of alternating current provides sufficient conditions responsible for nanosized materials formation (grain size: 20–30 nm for separate copper oxidation, 15–20 nm for joint copper and aluminum oxidation). In the present work, XRD and DSC/DTG, SEM analyses were used to characterize the electrolysis products. A decrease of the thermal transformation temperature (Cu2O oxidation at 260 °С, copper-aluminum layered double hydroxide (Cu-Al/LDH) decomposition at 140 °С) of the products of both separate copper oxidation and joint copper and aluminum (or cadmium) oxidation was established when compared with those of the same oxides prepared by the usual methods. Solution aging of these products after joint electrochemical oxidation of copper and aluminum results in the transformation of Cu2O-AlOOH to Cu-Al/LDH. Products of the joint oxidation of copper and cadmium consist of oxides and hydroxides of copper and cadmium (γ-Cd(OH)2, Cu(OH)2, β-Cd(OH)2, CdО and Cu2O), which are not capable to form LDH. The synthesized copper-containing mixed metal oxide will be investigated elsewhere to obtain their structural characterization and operating performance in catalytic, photocatalytic and other applications.
中文翻译:

金属非平衡电化学氧化制备纳米分散含铜粉末的热制备及表征
摘要 铜、铝和镉氧化物的物理化学性质决定了它们以不同方式应用。使用交流电 (AC) 进行电化学金属氧化会影响制备的含铜氧化物材料的带隙并导致蓝移。交流电的使用为纳米材料的形成提供了足够的条件(晶粒尺寸:20-30 nm 用于单独的铜氧化,15-20 nm 用于联合铜和铝氧化)。在目前的工作中,XRD 和 DSC/DTG、SEM 分析用于表征电解产物。热转变温度降低(Cu2O 氧化在 260 °С,铜铝层状双氢氧化物 (Cu-Al/LDH) 在 140 °С 分解,分别与铜氧化和铜铝(或镉)联合氧化的产物与相同的氧化物制备的氧化物进行比较。通常的方法。在铜和铝的联合电化学氧化后,这些产品的固溶老化导致 Cu2O-AlOOH 转变为 Cu-Al/LDH。铜和镉的联合氧化产物包括铜和镉的氧化物和氢氧化物(γ-Cd(OH)2、Cu(OH)2、β-Cd(OH)2、CdО 和 Cu2O),它们不能形成LDH。合成的含铜混合金属氧化物将在别处进行研究,以获得它们在催化、光催化和其他应用中的结构表征和操作性能。
更新日期:2020-10-01
中文翻译:

金属非平衡电化学氧化制备纳米分散含铜粉末的热制备及表征
摘要 铜、铝和镉氧化物的物理化学性质决定了它们以不同方式应用。使用交流电 (AC) 进行电化学金属氧化会影响制备的含铜氧化物材料的带隙并导致蓝移。交流电的使用为纳米材料的形成提供了足够的条件(晶粒尺寸:20-30 nm 用于单独的铜氧化,15-20 nm 用于联合铜和铝氧化)。在目前的工作中,XRD 和 DSC/DTG、SEM 分析用于表征电解产物。热转变温度降低(Cu2O 氧化在 260 °С,铜铝层状双氢氧化物 (Cu-Al/LDH) 在 140 °С 分解,分别与铜氧化和铜铝(或镉)联合氧化的产物与相同的氧化物制备的氧化物进行比较。通常的方法。在铜和铝的联合电化学氧化后,这些产品的固溶老化导致 Cu2O-AlOOH 转变为 Cu-Al/LDH。铜和镉的联合氧化产物包括铜和镉的氧化物和氢氧化物(γ-Cd(OH)2、Cu(OH)2、β-Cd(OH)2、CdО 和 Cu2O),它们不能形成LDH。合成的含铜混合金属氧化物将在别处进行研究,以获得它们在催化、光催化和其他应用中的结构表征和操作性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号