Separation and Purification Technology ( IF 8.1 ) Pub Date : 2020-09-25 , DOI: 10.1016/j.seppur.2020.117781 Guobi Li , Jing Chen , Baiqiao Song , Shaomin Wang , Rongkai Pan , Lingmin Pei , Shenggui Liu , Qingyuan Yang
|
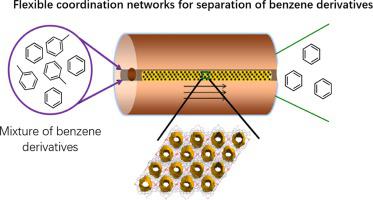
The separation of toluene derivatives from each other is an important, challenging, and energy exhaustive process in chemical industry. Here we report a new flexible coordination network, [Cu(4-pmntd)2(NO3)2](4-pmntd = N, N’-Bis(4-pyridymethy)naphthalene diimide), that exhibits not only selective separation of toluene over benzene and xylene, but also can straight extract toluene from water. The high performance for selective adsorption of toluene was validated by single-crystal XRD, vapor sorption isotherms, and NMR. The detailed structural information discloses that the naphthalene diimide derivative ligand can form intermolecular charge-transfer interactions with toluene, and thus highly selective capture toluene with its derivatives. Naphthalene diimide ligand engenders favorable aromatic π interactions for its electron-deficient and planar aromatic surface. The electron giving substituents on aromatic ring cause an overall change in the cloud density, which can be employed in separating toluene derivatives. When 1:1 toluene/benzene mixture pass through a fixed-bed packed column with this coordination network, 96% purity of benzene can be directly produced during the first adsorption cycle. The outlet xylene purity was raised from 50% to 85% when 1:1 toluene/xylene mixture pass through the porous materials. The separation performance of the coordination network could be used for industrial separation of toluene derivatives with a low energy cost at room temperature.
中文翻译:

基于柔性萘二酰亚胺配位网络的苯衍生物中甲苯的分离和水中甲苯的提取
甲苯衍生物彼此之间的分离是化学工业中重要的,具有挑战性的和耗能的过程。在这里,我们报告了一个新的灵活协调网络[Cu(4-pmntd)2(NO 3)2](4-pmntd = N,N'-双(4-吡啶甲基)萘二酰亚胺),不仅显示甲苯在苯和二甲苯上的选择性分离,而且还可以从水中直接萃取甲苯。通过单晶XRD,气相吸附等温线和NMR验证了甲苯选择性吸附的高性能。详细的结构信息公开了萘二酰亚胺衍生物配体可以与甲苯形成分子间的电荷转移相互作用,从而以其衍生物高度选择性地捕获甲苯。萘二酰亚胺配体为其缺电子且平面的芳族表面产生有利的芳族π相互作用。芳环上的给电子取代基引起云密度的总体变化,可用于分离甲苯衍生物。1时 1种甲苯/苯混合物通过具有这种配位网络的固定床填充柱,在第一个吸附循环中可以直接生产96%的苯纯度。当1∶1的甲苯/二甲苯混合物通过多孔材料时,出口二甲苯的纯度从50%提高到85%。该配位网络的分离性能可用于在室温下以较低的能源成本对甲苯衍生物进行工业分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号