Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-09-24 , DOI: 10.1016/j.jaci.2020.08.039 Pauline Azzano 1 , Maxime Paquin 2 , Alexandra Langlois 1 , Charles Morin 3 , Guy Parizeault 3 , Jonathan Lacombe-Barrios 1 , Kathryn Samaan 1 , François Graham 2 , Louis Paradis 4 , Anne Des Roches 1 , Philippe Bégin 5
|
Background
Omalizumab has been shown to improve the safety and feasibility of oral immunotherapy (OIT), but the optimal dosage strategy is unknown.
Objective
Our aim was to identify determinants of omalizumab dose–related efficacy in the context of OIT.
Methods
The study sample consisted of a clinical cohort of 181 patients treated with omalizumab-enabled oral immunotherapy at 3 centers. Patients received omalizumab for at least 2 months before an initial food escalation (IFE) with a mix of up to 6 allergens. Progression through IFE steps was assessed with survival analysis. Continued food dose tolerance with omalizumab weaning was also documented.
Results
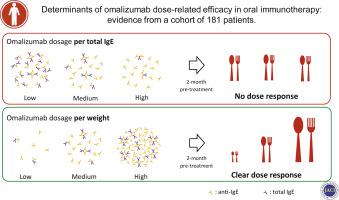
Omalizumab dosage per weight alone was strongly associated with progression through the IFE (χ2 = 28.18; P < .0001), whereas the standard dosage per weight and total IgE level used for asthma was not (χ2 = 0.001; P = .97). When the values at time of IFE were estimated through pharmacokinetics and pharmacodynamics simulation, IFE outcome was best predicted by a model that includes levels of free allergen-specific IgE and their interaction with blocking omalizumab-IgE complexes and free omalizumab levels in serum (χ2 = 65.84; degrees of freedom [df] = 2; P < .0005). The occurrence of immediate-type reactions to food dosing subsequent to weaning of omalizumab was associated with a greater ratio of specific IgE level to total IgE level at baseline (geometric mean 0.39 vs 0.16 in those without symptom; P < .0001).
Conclusion
In the context of OIT and IgE-mediated disease, omalizumab dosages should be adjusted for body weight alone, independently of total IgE level. The fraction of allergen-specific/total IgE may be useful to predict patients at greater risk of food dosing reactions subsequent to weaning.
中文翻译:

口服免疫疗法中与奥马珠单抗剂量相关的疗效的决定因素:来自181名患者的证据
背景
Omalizumab已被证明可以提高口服免疫疗法(OIT)的安全性和可行性,但最佳剂量策略尚不清楚。
目的
我们的目的是确定OIT背景下与奥马珠单抗剂量相关的疗效的决定因素。
方法
该研究样本由3个中心的181例接受奥马珠单抗支持的口服免疫疗法治疗的患者组成。在最初的食物升级(IFE)之前,患者应接受奥马珠单抗至少2个月,并混合多达6种过敏原。通过生存分析评估通过IFE步骤的进展。还记录了奥马珠单抗断奶后持续的食物剂量耐受性。
结果
每单独重量奥马珠单抗的剂量是紧密联系在一起的进展通过IFE相关联(χ 2 = 28.18; P <0.0001),而用于哮喘每重量和总IgE水平的标准剂量没有(χ 2 = 0.001; P = 0.97 )。当在IFE的时间的值是通过药动学和药效学模拟估计,IFE结果是最好通过包括无过敏原特异性IgE的水平及其与封闭奥马珠单抗特异性IgE配合物和在无血清奥马珠单抗水平相互作用的模型预测(χ 2 = 65.84;自由度[df] = 2;P <.0005)。断奶奥马珠单抗后对食物投加立即发生反应的情况与基线时特定IgE水平与总IgE水平的比率较高相关(无症状者的几何平均值为0.39 vs 0.16;P <.0001)。
结论
在OIT和IgE介导的疾病中,应单独针对体重调整奥马珠单抗的剂量,而与总IgE水平无关。过敏原特异性/总IgE的比例可能有助于预测断奶后食物剂量反应风险更高的患者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号