当前位置:
X-MOL 学术
›
Inorg. Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of SeO42- by delaminated Mg-Al layered double hydroxide nanosheets
Inorganic Chemistry Communications ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.inoche.2020.108266 Tomohito Kameda , Daichi Ikeda , Shogo Kumagai , Yuko Saito , Toshiaki Yoshioka
Inorganic Chemistry Communications ( IF 4.4 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.inoche.2020.108266 Tomohito Kameda , Daichi Ikeda , Shogo Kumagai , Yuko Saito , Toshiaki Yoshioka
|
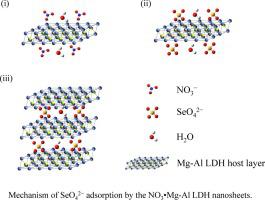
Abstract Layered double hydroxides (LDHs) are excellent anion-exchange materials, and are useful in adsorbing hazardous anions. The LDHs form nanosheets upon delamination, which improves their efficiency. In this study, we investigated the SeO42− adsorption properties of the nanosheets obtained by the delamination of NO3⋅Mg-Al LDH in an aqueous solution and compared them with those of the NO3⋅Mg-Al LDH. The SeO42− adsorption by both substances was a “pseudo-second-order rate reaction”. The reaction-rate constant during the early stage was greater for the nanosheets as their surface area was larger than the NO3⋅Mg-Al LDH. The amorphous nanosheets reverted to an ordered LDH structure after the anion exchange. Atomic force microscopy (AFM) imaging and the corresponding height profile analysis of the samples confirmed the nanosheets stacking post SeO42− adsorption. We propose a mechanism of the SeO42− adsorption by the NO3⋅Mg-Al LDH nanosheets. These findings may improve the understanding of the anion exchange by the LDHs, which may lead to the development of high-efficiency materials that extract metals such as Se from wastewater.
中文翻译:

分层Mg-Al层状双氢氧化物纳米片对SeO42-的吸附
摘要 层状双氢氧化物(LDHs)是优良的阴离子交换材料,可用于吸附有害阴离子。LDH 在分层时形成纳米片,这提高了它们的效率。在这项研究中,我们研究了通过 NO3·Mg-Al LDH 在水溶液中分层获得的纳米片的 SeO42− 吸附特性,并将它们与 NO3·Mg-Al LDH 的吸附特性进行了比较。两种物质对 SeO42− 的吸附是“伪二级速率反应”。纳米片在早期的反应速率常数更大,因为它们的表面积大于 NO3·Mg-Al LDH。阴离子交换后,无定形纳米片恢复到有序的LDH结构。样品的原子力显微镜 (AFM) 成像和相应的高度剖面分析证实了纳米片在 SeO42− 吸附后堆积。我们提出了 NO3·Mg-Al LDH 纳米片吸附 SeO42− 的机制。这些发现可能会提高对 LDH 阴离子交换的理解,这可能会导致开发从废水中提取硒等金属的高效材料。
更新日期:2020-12-01
中文翻译:

分层Mg-Al层状双氢氧化物纳米片对SeO42-的吸附
摘要 层状双氢氧化物(LDHs)是优良的阴离子交换材料,可用于吸附有害阴离子。LDH 在分层时形成纳米片,这提高了它们的效率。在这项研究中,我们研究了通过 NO3·Mg-Al LDH 在水溶液中分层获得的纳米片的 SeO42− 吸附特性,并将它们与 NO3·Mg-Al LDH 的吸附特性进行了比较。两种物质对 SeO42− 的吸附是“伪二级速率反应”。纳米片在早期的反应速率常数更大,因为它们的表面积大于 NO3·Mg-Al LDH。阴离子交换后,无定形纳米片恢复到有序的LDH结构。样品的原子力显微镜 (AFM) 成像和相应的高度剖面分析证实了纳米片在 SeO42− 吸附后堆积。我们提出了 NO3·Mg-Al LDH 纳米片吸附 SeO42− 的机制。这些发现可能会提高对 LDH 阴离子交换的理解,这可能会导致开发从废水中提取硒等金属的高效材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号