Developmental Cell ( IF 10.7 ) Pub Date : 2020-09-25 , DOI: 10.1016/j.devcel.2020.09.001 Agathe Chaigne 1 , Céline Labouesse 2 , Ian J White 1 , Meghan Agnew 1 , Edouard Hannezo 3 , Kevin J Chalut 2 , Ewa K Paluch 4
|
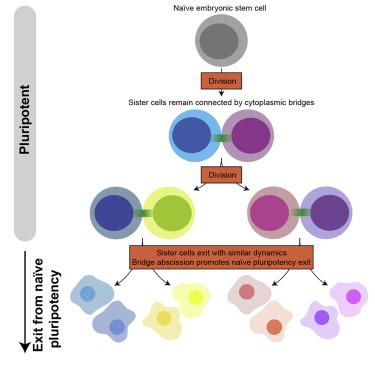
Cell fate transitions are key to development and homeostasis. It is thus essential to understand the cellular mechanisms controlling fate transitions. Cell division has been implicated in fate decisions in many stem cell types, including neuronal and epithelial progenitors. In other stem cells, such as embryonic stem (ES) cells, the role of division remains unclear. Here, we show that exit from naive pluripotency in mouse ES cells generally occurs after a division. We further show that exit timing is strongly correlated between sister cells, which remain connected by cytoplasmic bridges long after division, and that bridge abscission progressively accelerates as cells exit naive pluripotency. Finally, interfering with abscission impairs naive pluripotency exit, and artificially inducing abscission accelerates it. Altogether, our data indicate that a switch in the division machinery leading to faster abscission regulates pluripotency exit. Our study identifies abscission as a key cellular process coupling cell division to fate transitions.
中文翻译:

脱落将细胞分裂与胚胎干细胞命运结合起来
细胞命运转变是发育和体内平衡的关键。因此,了解控制命运转变的细胞机制至关重要。细胞分裂与许多干细胞类型的命运决定有关,包括神经元和上皮祖细胞。在其他干细胞中,例如胚胎干 (ES) 细胞,分裂的作用仍不清楚。在这里,我们表明小鼠 ES 细胞中幼稚多能性的退出通常发生在分裂后。我们进一步表明,姐妹细胞之间的退出时间密切相关,它们在分裂后很长时间仍然通过细胞质桥连接,并且随着细胞退出幼稚多能性,桥的脱落逐渐加速。最后,干扰脱落会损害幼稚多能性退出,而人为诱导脱落会加速它。共,我们的数据表明,导致更快脱落的分裂机制的转换调节了多能性退出。我们的研究将脱落确定为将细胞分裂与命运转变相结合的关键细胞过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号