当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The kinetic mechanism of acetylene hydrogenation to prepare ethane over FexOy clusters: A DFT study
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.ces.2020.116170 Ren Feng , Lun Pan , Fengwu Li , Daidi Xu , Ronghui Shi , Libo Dai , Cuicui Ding , Ji-Jun Zou , Min Zhang
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.ces.2020.116170 Ren Feng , Lun Pan , Fengwu Li , Daidi Xu , Ronghui Shi , Libo Dai , Cuicui Ding , Ji-Jun Zou , Min Zhang
|
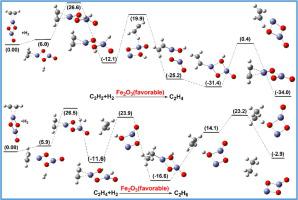
Abstract The acetylene hydrogenation reaction over FeO, Fe2O3 and Fe3O4 clusters are theoretically investigated, via C2H2 adsorption, approach of molecular H2 to cluster, H2 dissociation on Fe O bond, and the sequential addition reaction of H atom. Over FeO cluster, the extremely high barrier for the addition reaction of H atom adsorbed on O atom (of cluster) predicts the impossible acetylene hydrogenation. Over Fe2O3 cluster, the overall barriers for H2 dissociation, addition reaction of two hydrogen atom are respectively 26.6, 32 and 31.8 kcal·mol−1 during the acetylene hydrogenation to form ethylene and 26.5, 35.5 and 9.1 kcal·mol−1 for its further hydrogenation to produce ethane. During the further hydrogenation pathway, the migration of semi-hydrogenated product C2H3 requires extra energy of 30.7 kcal mol−1. These barriers are lower than those of the pathways over Fe3O4 cluster. Hence, among three FexOy clusters, hydrogenation of acetylene to produce ethane on Fe2O3 cluster is kinetically most favorable.
中文翻译:

FexOy 簇上乙炔加氢制备乙烷的动力学机制:DFT 研究
摘要 通过C2H2 吸附、分子H2 接近簇、H2 在Fe O 键上的解离和H 原子的顺序加成反应,从理论上研究了FeO、Fe2O3 和Fe3O4 簇上的乙炔加氢反应。在 FeO 团簇上,吸附在(团簇)O 原子上的 H 原子的加成反应的极高势垒预示着乙炔加氢是不可能的。在Fe2O3簇上,乙炔加氢生成乙烯时H2解离、两个氢原子加成反应的总势垒分别为26.6、32和31.8 kcal·mol-1,进一步形成乙烯的势垒分别为26.5、35.5和9.1 kcal·mol-1加氢生成乙烷。在进一步加氢过程中,半氢化产物 C2H3 的迁移需要 30.7 kcal mol-1 的额外能量。这些障碍低于 Fe3O4 簇上的路径。因此,在三个 FexOy 簇中,乙炔在 Fe2O3 簇上加氢生成乙烷在动力学上是最有利的。
更新日期:2021-02-01
中文翻译:

FexOy 簇上乙炔加氢制备乙烷的动力学机制:DFT 研究
摘要 通过C2H2 吸附、分子H2 接近簇、H2 在Fe O 键上的解离和H 原子的顺序加成反应,从理论上研究了FeO、Fe2O3 和Fe3O4 簇上的乙炔加氢反应。在 FeO 团簇上,吸附在(团簇)O 原子上的 H 原子的加成反应的极高势垒预示着乙炔加氢是不可能的。在Fe2O3簇上,乙炔加氢生成乙烯时H2解离、两个氢原子加成反应的总势垒分别为26.6、32和31.8 kcal·mol-1,进一步形成乙烯的势垒分别为26.5、35.5和9.1 kcal·mol-1加氢生成乙烷。在进一步加氢过程中,半氢化产物 C2H3 的迁移需要 30.7 kcal mol-1 的额外能量。这些障碍低于 Fe3O4 簇上的路径。因此,在三个 FexOy 簇中,乙炔在 Fe2O3 簇上加氢生成乙烷在动力学上是最有利的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号