Biosensors and Bioelectronics ( IF 10.7 ) Pub Date : 2020-09-25 , DOI: 10.1016/j.bios.2020.112648 Roberta D'Agata 1 , Noemi Bellassai 2 , Matteo Allegretti 3 , Andrea Rozzi 4 , Saša Korom 4 , Alex Manicardi 5 , Elisa Melucci 6 , Edoardo Pescarmona 6 , Roberto Corradini 4 , Patrizio Giacomini 3 , Giuseppe Spoto 1
|
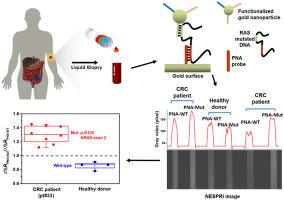
RAS mutations in the blood of colorectal cancer (CRC) patients are emerging as biomarkers of acquired resistance to Epidermal Growth Factor Receptor therapy. Unfortunately, reliable assays granting fast, real-time monitoring of treatment response, capable of refining retrospective, tissue-based analysis, are still needed. Recently, several methods for detecting blood RAS mutations have been proposed, generally relying on multi-step and PCR-based, time-consuming and cost-ineffective procedures. By exploiting a liquid biopsy approach, we developed an ultrasensitive nanoparticle-enhanced plasmonic method for detecting ~1 aM RAS single nucleotide variants (SNVs) in the plasma of CRC patients. The assay does not require the extraction of tumor DNA from plasma and detects it in volumes as low as 40 μL of plasma, which is at least an order of magnitude smaller than that required by state of the art liquid biopsy technologies. The most prevalent RAS mutations are detected in DNA from tumor tissue with 100% sensitivity and 83.33% specificity. Spike-in experiments in human plasma further encouraged assay application on clinical specimens. The assay was proven in plasma from CRC patients and healthy donors, and full discrimination between mutated DNA from patients over wild-type DNA from healthy volunteers was obtained thus demonstrating its promising avenue for cancer monitoring based on liquid biopsy.
中文翻译:

大肠癌患者循环 RAS 突变 DNA 的直接等离子体检测
结直肠癌 (CRC) 患者血液中的RAS突变正在成为获得性表皮生长因子受体治疗耐药的生物标志物。不幸的是,仍然需要能够快速、实时监测治疗反应、能够改进回顾性、基于组织的分析的可靠分析。最近,已经提出了几种检测血液RAS突变的方法,通常依赖于多步骤和基于 PCR 的、耗时且成本低效的程序。通过利用液体活检方法,我们开发了一种超灵敏的纳米粒子增强等离子体方法,用于检测~1 aM RASCRC 患者血浆中的单核苷酸变异体 (SNV)。该测定不需要从血浆中提取肿瘤 DNA,并且可以检测低至 40 μL 血浆的体积,这比最先进的液体活检技术所需的体积至少小一个数量级。在来自肿瘤组织的 DNA 中检测到最普遍的RAS突变,敏感性为 100%,特异性为 83.33%。人血浆中的掺入实验进一步鼓励了临床标本的分析应用。该测定在来自 CRC 患者和健康供体的血浆中得到证实,并且获得了来自患者的突变 DNA 与来自健康志愿者的野生型 DNA 的完全区分,从而证明了其基于液体活检的癌症监测的有希望的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号