Letters in Organic Chemistry ( IF 0.7 ) Pub Date : 2020-09-30 , DOI: 10.2174/1570178617666200129144750 Elmira Danaie 1 , Shiva Masoudi 1 , Nasrin Masnabadi 2
|
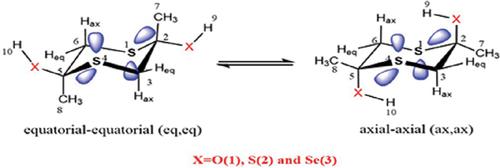
Conformational behaviors of 2,5-dimethyl-1,4-dithiane-2,5-diol (compound 1), 2,5- dimethyl-1,4-dithiane-2,5-dithiol (compound 2) and 2,5-dimethyl-1,4-dithiane-2,5-diselenol (compound 3) were investigated by the B3LYP/6-311+G **, the M06-2X/aug-ccpvdz levels of theory and natural bond orbital NBO analysis. The structures and the structural parameters of the mentioned molecules were optimized by the B3LYP and the M06-2X methods. We assessed the roles and contributions of the effective factors in the conformational properties of the mentioned compounds by means of the B3LYP and M06-2X levels of theory and the NBO interpretations. The stereoelectronic effects of the mentioned molecules were studied using the NBO analysis. The results showed that the stereoelectronic effects were in favor of the (ax,ax) conformers (the most stable conformations), from compound 1 to compound 3; therefore, these effects have impacts on the conformational properties of compounds 1-3, and stabilization energies associated with LP2X→ σ*S1-C2 electron delocalization, where [X= O, S, and Se], for 1-ax, ax conformer has the greatest value between all of the other conformers. Therefore, according to the calculated thermodynamic parameters, the stability of the 1-ax, ax compound was justified by the presence of LP2X→σ*S1-C2 electron delocalization. A molecular orbital explanation was conducted to investigate the correlations between the linear combinations of natural bond orbitals in the HOMOs, LUMOs and the molecular reactivity parameters. There is a direct relationship between the stereoelectronic effects, molecular reactivity and thermodynamic parameters of compounds 1 to 3 as the harder ax, ax conformations with the greater stereoelectronic effects and ΔG(eq-ax) values are more stable than their corresponding eq, eq conformers. Besides frontier molecular orbitals (FMOs), mapped molecular electrostatic potential (MEP) surfaces of conformations of compounds 1 to 3 were investigated.
中文翻译:

2,5-二甲基-1,4-二巯基-2,5-二醇与类似S和Se的构象行为的计算研究:DFT和NBO研究
2,5-二甲基-1,4-二硫代-2,5-二醇(化合物1),2,5-二甲基-1,4-二硫代-2,5-二硫醇(化合物2)和2,5的构象行为通过B3LYP / 6-311 + G **,M06-2X / aug-ccpvdz水平的理论值和自然键轨道NBO分析研究了-二甲基-1,4-二硫杂环丁-2,5-二甲苯酚(化合物3)。通过B3LYP和M06-2X方法优化了上述分子的结构和结构参数。我们通过理论的B3LYP和M06-2X水平以及NBO解释,评估了有效因子在上述化合物的构象性质中的作用和贡献。使用NBO分析研究了上述分子的立体电子效应。结果表明,立体电子效应有利于(ax,ax)构象异构体(最稳定的构象),从化合物1到化合物3;因此,这些效应对化合物1-3的构象性质以及与LP2X→σ* S1-C2电子离域相关的稳定能(其中[X = O,S和Se],对于1-ax,ax构象异构体)有影响。在所有其他符合者中具有最大的价值。因此,根据计算出的热力学参数,通过存在LP2X→σ* S1-C2电子离域可以证明1-ax,ax化合物的稳定性。进行了分子轨道解释,以研究HOMO,LUMO中自然键轨道的线性组合与分子反应性参数之间的相关性。化合物1至3(较硬的斧头)的立体电子效应,分子反应性和热力学参数之间存在直接关系,(eq-ax)值比其相应的eq,eq构象异构体更稳定。除了前沿分子轨道(FMO),还研究了化合物1-3构象的映射分子静电势(MEP)表面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号