Current Analytical Chemistry ( IF 1.8 ) Pub Date : 2021-08-31 , DOI: 10.2174/1573411016666200106101921 Nady A. Fathy 1 , Sahar M. El-Khouly 1 , Ola I. El-Shafey 2
|
Background: Application of nanotechnology in wastewater treatment is the solution to cope with the conflict between people’s increasing demand for water and the world-wide water shortage. The main goal of this study is to prepare modified carbon nanostructures (CNSs) from sugarcane bagasse waste (SCB) as effective adsorbents for removing toxic Cr(VI) ions from their aqueous solutions.
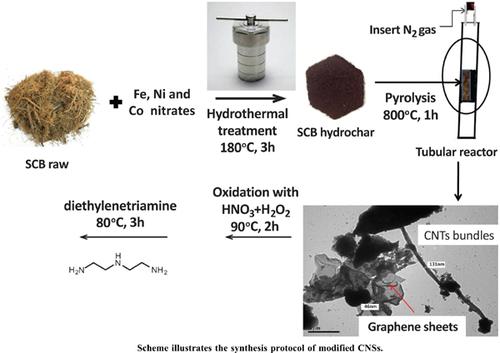
Methods: The preparation of CNSs was performed via catalytic hydrothermal/carbonization of SCB. The resultant CNSs sample was oxidized by oxidation with HNO3/H2O2 (O-CNSs) and then followed by coating with diethylenetriamine (N-CNSs). Transmission electron microscope (TEM), scanning electron microscope attached to energy-dispersive X-ray (SEM/EDX), fourier transform infrared (FTIR) and nitrogen adsorption analyses were used to determine the morphology and surface properties of CNSs. Adsorption and desorption studies of Cr(VI) ions onto these modified CNSs were investigated. Effects of initial concentration of Cr(VI), pH of solution and temperature in the batch mode were estimated. Adsorption studies were analyzed by Langmuir, Freundlich and Dubinin- Radushkevich models. Pseudo-first-order, pseudo-second-order and intraparticle diffusion models were undertaken to follow the adsorption mechanism.
Results: The prepared samples composed of carbon nanotubes and graphene sheets were evident by TEM and SEM. The adsorption of Cr(VI) is exothermic in nature, well-fitted with Langmuir, described by pseudo-second order kinetic model and then is uncontrolled by intraparticle diffusion step. It was found that the adsorption of Cr(VI) was higher uptake over O-CNSs (56 mg/g) than that by NCNSs (44 mg/g). This was ascribed to that the first sample is enriched with acidic O-functional groups and possessed higher specific surface area (188 m2/g). Desorption studies were achieved by HNO3 and NaOH reagents for recovering the Cr(VI) from O-CNSs. Results revealed that about 90% of Cr(VI) can be recovered by HNO3 more than that by NaOH till the third run.
Conclusion: Two modified CNSs samples were successfully prepared from SCB. Adsorption of Cr(VI) is highly relied on initial concentration of Cr(VI), pH and temperature. The main factors controlling the adsorption behavior of Cr(VI) are the acidic functional groups and the accessible surface area on O-CNSs. Furthermore, the O-CNSs attained high stability in recycling tests for Cr(VI) removal.
中文翻译:

甘蔗渣水炭改性碳纳米结构处理铬污染水体
背景:纳米技术在污水处理中的应用是解决人们日益增长的用水需求与世界范围内水资源短缺之间冲突的解决方案。本研究的主要目标是从甘蔗渣废物 (SCB) 中制备改性碳纳米结构 (CNS) 作为有效吸附剂,用于从水溶液中去除有毒的 Cr(VI) 离子。
方法:通过SCB的催化水热/碳化制备CNSs。用 HNO 3 /H 2 O 2氧化得到的 CNSs 样品(O-CNSs),然后用二亚乙基三胺 (N-CNSs) 包被。使用透射电子显微镜 (TEM)、能量色散 X 射线扫描电子显微镜 (SEM/EDX)、傅立叶变换红外 (FTIR) 和氮吸附分析来确定 CNS 的形貌和表面性质。研究了 Cr(VI) 离子在这些改性 CNS 上的吸附和解吸研究。评估了分批模式中 Cr(VI) 的初始浓度、溶液的 pH 值和温度的影响。吸附研究通过 Langmuir、Freundlich 和 Dubinin-Radushkevich 模型进行分析。进行了伪一级、伪二级和颗粒内扩散模型以遵循吸附机制。
结果:制备的由碳纳米管和石墨烯片组成的样品在透射电镜和扫描电镜下清晰可见。Cr(VI) 的吸附本质上是放热的,与 Langmuir 很好地拟合,由伪二级动力学模型描述,然后不受颗粒内扩散步骤的控制。结果表明,O-CNSs(56 mg/g)对Cr(VI)的吸附高于NCNSs(44 mg/g)的吸附。这归因于第一个样品富含酸性O-官能团并具有更高的比表面积(188 m 2 /g)。解吸研究是通过 HNO 3和 NaOH 试剂实现的,用于从 O-CNS 中回收 Cr(VI)。结果表明,直到第三次运行,HNO 3可以比NaOH回收约90%的Cr(VI)。
结论:从 SCB 成功制备了两个修饰的 CNSs 样品。Cr(VI) 的吸附高度依赖于 Cr(VI) 的初始浓度、pH 值和温度。控制 Cr(VI) 吸附行为的主要因素是酸性官能团和 O-CNS 上的可及表面积。此外,O-CNSs 在去除 Cr(VI) 的回收测试中获得了高稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号