Current Analytical Chemistry ( IF 1.7 ) Pub Date : 2021-08-31 , DOI: 10.2174/1573411016666200123143516 Bathinapatla Ayyappa 1 , Suvardhan Kanchi 2 , Myalowenkosi I. Sabela 2 , Krishna Bisetty 3
|
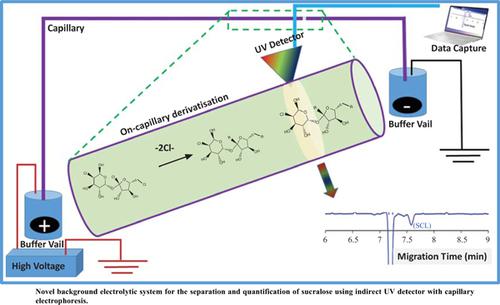
Background: Sucralose is a high intensity artificial sweetener and chemically known as 1,6-dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside. It is used as a sweetener and flavour enhancer in foods and beverages. Due to its high stability at wider temperatures and pH, it made its applicability in various food products throughout the world. As per Joint FAO/WHO Expert Group on Food Additives (JECFA) in 1990, the daily intake of sucralose is 0-15 mg/kg body weight. The literature reports suggest that sucralose has a possible health threat due to the presence of chlorine groups, thereby leading to several illnesses. The growing interest in the use of sucralose (SCL) in the foods makes it necessary in developing a fast, reliable, cost effective and reproducible analytical method to determine SCL in food samples. The detection of sucralose and other carbohydrates like fructose, glucose and sucrose is a challenging task owing to its: (i) unavailability of the charged functions and (ii) lack of absorption of strong chromophoric nature in the UV region. Therefore, separation of non-absorbing neutral molecules needs a careful procedure with suitable electrolyte systems.
Methodology: An indirect UV detection capillary electrophoretic method is described for the separation of sucralose in different food samples. It was achieved by nucleophile substitution (SN2) in the presence of amine as background electrolytes. The morpholine buffer showed good buffering capacity in terms of migration time (< 8.0 min) and baseline stability when compared to other amine buffers (ethylamine, piperidine, triethylamine). The analytical applications of proposed method showed by recovery percentages of sucralose in real and spiked samples on intra and inter-day basis at optimum experimental conditions of 0.2 M buffer concentration and pH 12.0 at 230 nm UV detection.
Results: The selection of BGE, UV detection wavelength, buffer concentration, buffer pH, cassette temperature and applied voltage was optimized to enhance the sensitivity and selectivity of the separation method. Recoveries obtained were ranging from 96.87 to 98.82% for real samples and 94.45 to 98.06% for spiked samples, respectively. Linearity was studied in the range of 2-10 mM, and showed a correlation coefficient of 0.9942. LOD and LOQ were found to be 0.3804 mg L-1 and 1.5215 mg L-1 with % RSD (n = 5) ± 1.27 and 1.19% with respect to migration time and peak area. Furthermore, to better understand the separation of sucralose with amine buffers, were investigated computationally using HOMO-LUMO calculations. The obtained results showed that the band gap decreases in the presence of amine moiety irrespective of its nature.
Conclusion: In the study, a novel background electrolytic system was successfully applied to separate sucralose using indirect UV detector with capillary electrophoresis. The FTIR results confirmed that the interaction of sucralose with different amine buffers to better understand the separation chemistry behind sucralose and amine complexes. Moreover, computational results indicate that the direction of charge transfer from the amine functionality to the glucofuranosyl ring in each amine derivative of sucralose confirms the strong interaction between sucralose and amines, which led to the baseline separation of sucralose in different food samples.
中文翻译:

使用胺作为背景电解质分离食品样品中的三氯蔗糖,并支持 DFT 计算
背景:三氯蔗糖是一种高强度人造甜味剂,化学上称为 1,6-dichloro-1,6-dideoxy-β-D-fructofuranosyl-4-chloro-4-deoxy-α-D-galactopyranoside。它在食品和饮料中用作甜味剂和增味剂。由于它在更宽的温度和 pH 值下具有高稳定性,因此它适用于世界各地的各种食品。根据 1990 年联合国粮农组织/世界卫生组织联合食品添加剂专家组 (JECFA),三氯蔗糖的每日摄入量为 0-15 毫克/公斤体重。文献报告表明,由于氯基团的存在,三氯蔗糖可能对健康构成威胁,从而导致多种疾病。人们对在食品中使用三氯蔗糖 (SCL) 的兴趣日益浓厚,因此有必要开发一种快速、可靠、具有成本效益且可重现的分析方法来测定食品样品中的 SCL。三氯蔗糖和其他碳水化合物(如果糖、葡萄糖和蔗糖)的检测是一项具有挑战性的任务,因为它们:(i)带电功能不可用和(ii)在紫外线区域缺乏强发色团性质的吸收。因此,非吸收性中性分子的分离需要使用合适的电解质系统进行仔细的程序。
方法:描述了一种用于分离不同食品样品中三氯蔗糖的间接紫外检测毛细管电泳方法。它是在胺作为背景电解质的情况下通过亲核取代 (S N 2) 实现的。与其他胺缓冲液(乙胺、哌啶、三乙胺)相比,吗啉缓冲液在迁移时间(< 8.0 分钟)和基线稳定性方面显示出良好的缓冲能力。所提出方法的分析应用表明,在 0.2 M 缓冲液浓度和 pH 12.0 的最佳实验条件下,在 230 nm 紫外检测下,真实样品和加标样品中三氯蔗糖的回收百分比在日内和日间基础上。
结果:优化了 BGE、UV 检测波长、缓冲液浓度、缓冲液 pH、盒温度和施加电压的选择,以提高分离方法的灵敏度和选择性。实际样品的回收率分别为 96.87 至 98.82%,加标样品的回收率分别为 94.45 至 98.06%。在 2-10 mM 范围内研究了线性,并显示相关系数为 0.9942。LOD 和 LOQ 分别为 0.3804 mg L -1和 1.5215 mg L -1相对于迁移时间和峰面积,% RSD (n = 5) ± 1.27 和 1.19%。此外,为了更好地理解三氯蔗糖与胺缓冲液的分离,使用 HOMO-LUMO 计算进行了计算研究。所得结果表明,无论其性质如何,在胺部分存在的情况下,带隙都会减小。
结论:在本研究中,使用带毛细管电泳的间接紫外检测器成功地应用了一种新型背景电解系统来分离三氯蔗糖。FTIR 结果证实三氯蔗糖与不同胺缓冲液的相互作用可以更好地了解三氯蔗糖和胺复合物背后的分离化学。此外,计算结果表明,在三氯蔗糖的每个胺衍生物中,电荷从胺官能团转移到呋喃糖基环的方向证实了三氯蔗糖和胺之间的强相互作用,这导致了不同食品样品中三氯蔗糖的基线分离。











































 京公网安备 11010802027423号
京公网安备 11010802027423号