Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2020-12-31 , DOI: 10.2174/1573412915666190522081113 Shun Liu 1 , Xun Wang 1 , Kaiping Zou 1 , Wei Liu 1 , Cunyu Li 2 , Yunfeng Zheng 2 , Qinmei Zhou 1 , Guoping Peng 2
|
Background: Zishen Tongguan (ZSTG) capsules were prepared at the Affiliated Hospital of Nanjing University of Chinese Medicine and have been proven to be clinically effective for treating pyelonephritis and benign prostatic hyperplasia. However, the quality standards are not ideal; a comprehensive study of the “quality markers” (Q-markers), the chemicals inherent in traditional Chinese medicine and its preparations, has not been carried out.
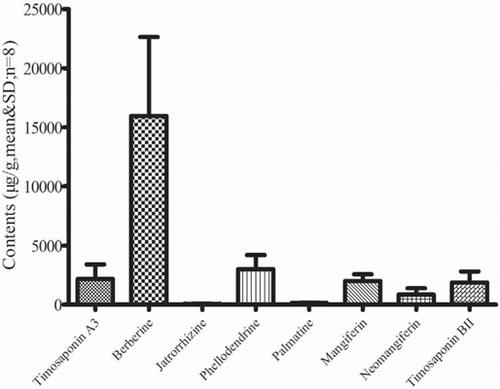
Experimental Methods: In this paper, a sensitive and specific ultra-high-performance liquid chromatographictandem mass spectrometry (UHPLC-MS/MS) method was developed for the simultaneous determination of eight potential Q-markers of ZSTG, including timosaponin A3, berberine, jatrorrhizine, phellodendrine, palmatine, mangiferin, neomangiferin, and timosaponin BII. A Kromasil 100-3.5 C18 column was used with a mobile phase of 0.2% formic acid with acetonitrile, and gradient elution at a flow rate of 0.2 mL/min was achieved in 13 minutes and used for separation. Detection was performed in positive/negative mode with multiple reaction monitoring (MRM).
Results: The analytical method was validated in terms of the sensitivity, linearity, accuracy, precision, repeatability, stability and recovery. The method established here was successfully applied to study the potential Q-markers in 8 batches of commercial samples, which demonstrated its use in improving the quality control of ZSTG.
Conclusion: The developed method had high repeatability and accuracy and was suitable for the simultaneous analysis of multiple Q-markers, which may provide a new basis for the comprehensive assessment and overall quality control of ZSTG.
中文翻译:

基于UHPLC-MS / MS同时测定紫参Tong关胶囊中8种潜在Q标记
背景:紫神Tong关(ZSTG)胶囊是在南京中医药大学附属医院制备的,已被证明可有效治疗肾盂肾炎和前列腺增生。但是,质量标准并不理想。尚未对“质量标志”(Q标志),中药及其制剂中固有的化学物质进行全面研究。
实验方法:本文建立了灵敏,特异的超高效液相色谱串联质谱法(UHPLC-MS / MS),用于同时测定ZSTG的8种潜在Q标记,其中包括timosaponin A3,小ber碱,香根草碱,黄柏碱,棕榈碱,芒果苷,新芒果苷和timosaponin BII。使用Kromasil 100-3.5 C18色谱柱,流动相为0.2%甲酸和乙腈,在13分钟内以0.2 mL / min的流速进行梯度洗脱,并将其用于分离。通过多反应监测(MRM)以阳性/阴性模式进行检测。
结果:该分析方法在灵敏度,线性,准确性,精密度,可重复性,稳定性和回收率方面均得到验证。本文建立的方法已成功用于研究8批次商业样品中潜在的Q标记,证明了其在改善ZSTG的质量控制中的用途。
结论:所建立的方法具有较高的重复性和准确性,适用于同时分析多个Q-marks,为ZSTG的综合评价和总体质量控制提供了新的依据。









































 京公网安备 11010802027423号
京公网安备 11010802027423号