Medicinal Chemistry ( IF 1.9 ) Pub Date : 2020-10-31 , DOI: 10.2174/1573406415666190826161123 Mahdieh Safakish 1 , Zahra Hajimahdi 1 , Rouhollah Vahabpour 2 , Rezvan Zabihollahi 2 , Afshin Zarghi 1
|
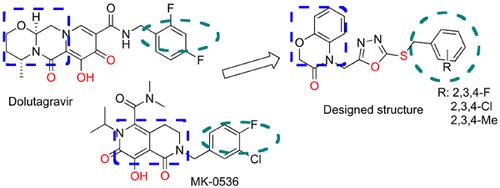
Introduction: Integrase is a validated drug target for anti-HIV-1 therapy. The second generation integrase inhibitors display π-stacking interaction ability with 3’-end nucleotide as a streamlined metal chelating pharmacophore.
Methods: In this study, we introduced benzoxazin-3-one scaffold for integrase inhibitory potential as bioisostere replacement strategy of 2-benzoxazolinone.
Results: Molecular modeling studies revealed that amide functionality alongside oxadiazole heteroatoms and sulfur in the second position of oxadiazole ring could mimic the metal chelating pharmacophore. The halobenzyl ring occupies hydrophobic site created by the cytidylate nucleotide (DC-16).
Conclusion: The most potent and selective compound displayed 110 μM IC50 with a selectivity index of more than 2.
中文翻译:

新型Benzoxazin-3-one衍生物:设计,合成,分子建模,抗HIV-1和整合酶抑制试验
简介:整合酶是一种经过验证的抗HIV-1治疗药物。第二代整合酶抑制剂表现出与3'-末端核苷酸的π堆积相互作用能力,这是一种简化的金属螯合药效团。
方法:在这项研究中,我们引入了具有整合酶抑制潜力的苯并恶嗪-3-one支架作为2-苯并恶唑啉酮的生物等排替代策略。
结果:分子建模研究表明,酰胺的功能以及恶二唑杂原子和硫在恶二唑环的第二位可以模拟金属螯合药效团。卤代苄基环占据由胞苷酸核苷酸(DC-16)产生的疏水位点。
结论:最有力和选择性的化合物显示出110μM的IC50,选择性指数大于2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号