Mini-Reviews in Organic Chemistry ( IF 1.9 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570193x17666200102105440 Nader Ghaffari Khaligh 1 , Mohd Rafie Johan 1
|
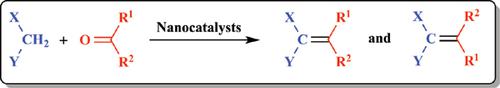
α,β-Unsaturated acids are well-known and useful reagents, and they have been applied in different fields due to their fascinating properties. The catalytic Knoevenagel condensation reaction is one of the most remarkable methods for the formation of C=C bonds. The multi-substituted alkenes can be obtained from the reaction of carbonyl and active methylene compounds in the presence of base catalysts, Brönsted catalysts, Lewis acid catalysts, or ionic liquids. In terms of providing both desirable structural diversity and compound libraries, Doebner-Knoevenagel condensation is the most efficient strategy. There is a high demand for an efficient, rapid, environment-friendly, and sustainable catalytic protocol under milder conditions for the stereoselective synthesis of Knoevenagel products, which can tolerate a wide variety of functions. Carrying out the transformations through alternative reagents, catalysts, or methods provides a valuable and broad space for selectivity. Herein, the recent advances in the synthesis of structurally diversified Knoevenagel products using nanocatalysts are reviewed.
中文翻译:

纳米催化Knoevenagel缩合的最新进展
α,β-不饱和酸是众所周知的有用试剂,并且由于其引人入胜的性能而被应用于不同领域。催化的Knoevenagel缩合反应是形成C = C键的最引人注目的方法之一。可以在碱催化剂,布朗斯台德催化剂,路易斯酸催化剂或离子液体的存在下,由羰基化合物与活性亚甲基化合物的反应获得多取代烯烃。就提供所需的结构多样性和化合物文库而言,Doebner-Knoevenagel缩合是最有效的策略。迫切需要在温和条件下有效,快速,环境友好和可持续的催化方案,以选择性合成Knoevenagel产品的立体选择性,从而可以耐受多种功能。通过替代试剂,催化剂或方法进行转化为选择性提供了宝贵而广阔的空间。在此,综述了使用纳米催化剂合成结构多样化的Knoevenagel产物的最新进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号