Medicinal Chemistry ( IF 1.9 ) Pub Date : 2021-05-31 , DOI: 10.2174/1573406416666191216121442 Rakesh Devidas Amrutkar 1 , Mahendra Sing Ranawat 2
|
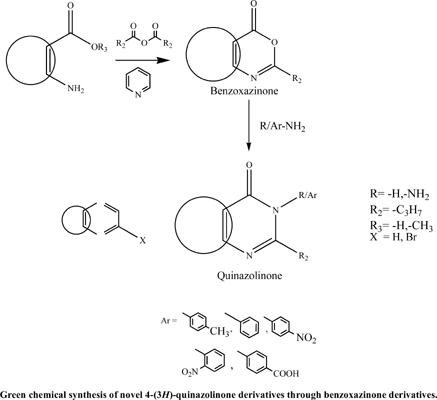
Background: Quinazolines and quinazolinones constitute a major class of biologically active molecules, both from natural and synthetic sources. The quinazolinone moiety is an important pharmacophore showing many types of pharmacological activities as shown in recent exhaustive review on the chemistry of 2-heteroaryl & heteroalkyl-4-quinazolinones4-quinazolinones that are the formal condensation products of anthranilic acid and amides. They can also be prepared in this fashion through the Niementowski quinazolinone synthesis, named after it’s discoverer Stefan Niementowski. Quinazoline and condensed quinazoline exhibit potent central nervous system (CNS) activities like anti-anxiety, analgesic, anti-inflammatory and anticonvulsant. Quinazolin-4- ones with 2, 3-disubstitution is reported to possess significant analgesic, anti-inflammatory and anticonvulsant activities.
Methods: To expand these views and application profiles, efforts have been made for the synthesis of a new class of quinazolinone by incorporating different amines into synthesized benzoxazinone ring by replacing O atom in the ring. Up till now, a great number of various procedures have been proposed for the synthesis of quinazolin-4-ones in the past few years. Using microwave radiation, this reaction could be easily and rapidly performed in very good yields, providing a large number of various 3-substituted-2- propyl-quinazolin-4-one derivatives which can be employed as useful bioactive compounds. We report a facile and efficient method for the synthesis of 3-substituted-2- propyl-quinazolin-4-one by the condensation reaction of anthranilic acid or halogen substituted anthranilic acid or methyl anthranilate, butanoic anhydride with various amines. We also report a drug/ligand or receptor/protein interactions by identifying the suitable active sites in the human gamma-aminobutyric acid receptor, the gaba (a)r-beta3 homopentamer human gammaaminobutyric acid receptor, and the gaba (a)r-beta3 homopentamer protein.
Results: It was observed in the reaction, 3-alkyl/aryl-2-alkyl-quinazolin-4-one gave good yield as well as good quality of the product by using MW. All the synthesized compounds were subjected to grid-based molecular docking studies. The results show that compound 4t has good affinity to the active site residue of the human gamma-aminobutyric acid receptor, and the gaba (a)r-beta3 homopentamer.
Conclusion: The Microwave irradiation for the synthesis of the title compounds offers a reduction in reaction time, operation simplicity, cleaner reaction, easy work-up and improved yields. The procedure clearly highlights the advantages of green chemistry. The data reported in this article may be helpful for the medicinal chemists who are working in this area. The protein-ligand interaction plays a significant role in structural based drug designing. In the present work, we have docked the ligand, 2, 3-disubstituted quinazolinone with the proteins that are used as the target for GABA-A receptor.
中文翻译:

某些 4-(3H)-喹唑啉酮衍生物作为人伽马-氨基丁酸受体 GABA (A)R-BETA3 同五聚体抑制剂的微波辅助合成和分子对接研究
背景:喹唑啉和喹唑啉酮构成了一类主要的生物活性分子,包括天然和合成来源。喹唑啉酮部分是一种重要的药效团,显示出许多类型的药理活性,如最近对 2-杂芳基和杂烷基-4-喹唑啉酮 4-喹唑啉酮(邻氨基苯甲酸和酰胺的正式缩合产物)的化学的详尽综述所示。它们也可以通过 Niementowski 喹唑啉酮合成以这种方式制备,该合成以其发现者 Stefan Niementowski 的名字命名。喹唑啉和缩合喹唑啉表现出有效的中枢神经系统 (CNS) 活性,如抗焦虑、镇痛、抗炎和抗惊厥。据报道,Quinazolin-4- 具有 2, 3-二取代具有显着的镇痛作用,
方法:为了扩展这些观点和应用概况,通过取代环中的 O 原子将不同的胺并入合成的苯并恶嗪酮环中,已经努力合成一类新的喹唑啉酮。迄今为止,在过去的几年中,人们提出了大量不同的合成喹唑啉-4-酮的方法。使用微波辐射,该反应可以以非常好的产率轻松快速地进行,提供大量各种 3-取代-2-丙基-喹唑啉-4-酮衍生物,可用作有用的生物活性化合物。我们报告了一种通过邻氨基苯甲酸或卤素取代邻氨基苯甲酸或邻氨基苯甲酸甲酯的缩合反应合成 3-取代-2-丙基-喹唑啉-4-one 的简便有效的方法,丁酸酐与各种胺。我们还通过鉴定人 γ-氨基丁酸受体、gaba (a)r-beta3 同五聚体人 γ-氨基丁酸受体和 gaba (a)r-beta3 中合适的活性位点来报告药物/配体或受体/蛋白质相互作用同源五聚体蛋白。
结果:在反应中观察到,3-烷基/芳基-2-烷基-喹唑啉-4-酮采用MW获得了良好的收率和产品质量。所有合成的化合物都进行了基于网格的分子对接研究。结果表明,化合物4t对人γ-氨基丁酸受体的活性位点残基和gaba(a)r-beta3同五聚体具有良好的亲和力。
结论:用于合成标题化合物的微波辐射缩短了反应时间、操作简单、反应更清洁、易于后处理并提高了产率。该程序清楚地突出了绿色化学的优势。本文报道的数据可能对从事该领域工作的药物化学家有所帮助。蛋白质-配体相互作用在基于结构的药物设计中起着重要作用。在目前的工作中,我们将配体 2, 3-二取代喹唑啉酮与用作 GABA-A 受体靶标的蛋白质对接。











































 京公网安备 11010802027423号
京公网安备 11010802027423号